Tab J - Human Health Benchmarks
A. OVERVIEW
As stated in the health risk assessment (HRA; Tab D), it is possible to conduct toxicity assessment and risk characterization using a "toxicological approach," and/or an "epidemiological approach." Tab D focused mainly on the toxicological approach; this section focuses on the epidemiological approach. This section is a comparison of human health effects found in the literature with dose estimates from the exposure assessment (EA) portion of the HRA. It supplements means to other sections of the HRA in judging the potential significance of the pesticide exposures estimated for Gulf War veterans. This section can also be called a "human toxicological approach," because it relies on a variety of human toxicology studies described later. Human toxicological data can be of variable quality because scientific gaps exist in exposure and effect information. The goal of this section is to answer the following core questions using available data from human studies:
This section provides the results of a literature search on the toxicological benchmarks for human health effects from exposure to the 12 pesticide active ingredients examined in the HRA. Literature values are then compared directly with exposure levels estimated in the HRA (see Tab D) to determine the likelihood of adverse health effects.
Literature research criteria were limited to human health effects data regarding exposure to pesticide active ingredients. Unless human experience was part of the health effect or toxicity endpoint decision, the literature search was purposely exclusive of any animal or plant data. Qualitative data regarding human metabolism and health effects were included in the summaries if deemed useful to answering the core question about human toxicity benchmarks.
Human dose-response data for exposure to pesticide active ingredients came from a variety of human toxicology studies including epidemiological, occupational, medical incident and controlled laboratory settings. Such data were the prime sources sought in the scientific literature for all 12 pesticide active ingredients of interest in this study. In some instances doses are estimated when plausible and when actual measured doses for humans were unavailable. If doses were estimated for human health effects found in the literature, the estimate assumptions were reported.
Human health effects data were sought for oral, dermal, inhalation and multiple routes for acute, subchronic and chronic exposures. All human health effect doses found in the literature, or their absence, for all exposure routes and scenarios were described in this report and its accompanying tables. All supporting references for health effects were cited.
Estimated Gulf War Veteran exposures, as summarized in the HRA, were compared directly to the documented human health effect benchmarks (HHEB). Uncertainty and safety factors applied by various organizations, such as EPA were not applied in this report. Elsewhere in the HRA, non-cancer and cancer reference doses based upon the toxicological approach, incorporating animal data and a variety of intraspecies and interspecies uncertainty factors, are compared with HRA dose estimates. To assist with comparing HHEB with the HRA exposure estimates, direct comparisons without any uncertainty factors were employed.
The scientific literature search, while comprehensive, was by no means exhaustive. It encompasses a 2-month search process covering a significant portion of the available and published scientific literature for human health effects. The possibility exists for proprietary research, unpublished research, manufacturer human experience data, or information about human health effects from data sources unavailable or uncovered during the course of the study to provide more complete information on human health effects from exposure to pesticide active ingredients.
B. DEET (N, N-Diethyl-m-Toluamide)
1. Acute Health Effects
a. Oral Exposure
In humans, a study was conducted on a number of cases of toxic reactions after ingestion of insect repellents containing DEET. The first case study involved a 14-year-old Canadian girl who had ingested the contents of a 50-mL bottle of insect repellent (95 percent DEET, 5 percent related toluamides). Within 30 minutes of being taken into custody, she was unconscious. Upon arrival at the hospital, mechanical ventilation was instituted. Approximately 90 minutes later, she had a generalized seizure that was successfully treated. Tremors persisted for the next 2 hours. Extensive clinical investigations uncovered no other etiology for her illness. At discharge 10 days after admission, she was physically and neurologically normal.
The second case study involved a 1-year-old girl whose 3�-year-old brother fed her approximately 25 mL of a 50-ml bottle of insect repellent (47.5 percent DEET, 2.5 percent toluamides). While en route to the hospital, the child had a brief (less than 1 minute) seizure. During her 40-minute stay in the emergency room, the child experienced four similar clonic spells. Extensive clinical investigations uncovered no other etiology for her illness. The following 20 hours in the hospital were uneventful, after which the child was discharged to go home.
Within the overall study (two of which have been detailed), each human patient had ingested large amounts of concentrated (47.5 percent-95 percent) insect repellent product. Their common symptoms and signs were coma, seizures, and hypotension, all occurring within 1 hour of ingestion of the insect repellent product. Two of the subjects died. The study concluded that the "ingestion of DEET can produce severe toxic reactions of rapid onset that may be fatal in some instances."[822]
One patient was documented as having ingested 8 oz of DEET in a successful suicide. Symptoms included cardiorespiratory arrest and status epilepticus.[823] For an acute oral exposure, this ingestion indicates an exposure level of 2400 mg/kg/d.
In other cases, urticarial skin reactions or gastrointestinal problems following ingestion were experienced. Also, neurotoxicity was reported in young children after prolonged dermal use or after appreciable ingestion, which was manifested as an encephalopathy with anxiety, behavioral changes, abnormal movements, lethargy, ataxia, mental confusion, seizures, and coma. DEET was detected in the blood of human volunteers within 2 hours of application on the volar surface of the foreman, and there was little evaporation from the skin. In one study, 48 percent of the dermally applied dose was absorbed within 6 hours. DEET is excreted primarily in the urine, and levels of DEET in body fat and the sciatic nerve were found to be higher than levels in plasma.[824]
b. Dermal Exposure
Between 1989 and 1995, the New York State Department of Health was notified of three cases of seizure related to dermal application of DEET. Two of the cases involved children: a 3-year-old girl and a 2-year-old boy. Both of the children recovered, although the girl reportedly went into cardiac arrest and was revived with CPR. The third case involved an adult male who applied the product twice, had a seizure, and died from choking on the food he was eating at the time of the seizure. Two annual reports were submitted by the DEET Joint Venture/Chemical Specialties Manufacturers Association covering all cases reported in 1995 and 1996. In 1995 and 1996, 32 cases mentioned some type of seizure activity that could not be ascribed to another likely cause based on the data initially collected. A number of the cases reported in 1995 and 1996 are still being followed to obtain medical records and complete the supplemental questionnaire forms. When the review is completed, it might be revealed that health effects occurred from causes other than DEET exposure.[825]
In a human dermal absorption study by the US Environmental Protection Agency, radiolabeled DEET in either 15 percent ethanol solution (12 mg; 36 �Ci) or undiluted (15 mg; 37 �CI) was dermally applied to two groups of healthy human volunteers (6 males/group; ages ranging from 20-29 years). The test material was applied on an area of 4 � 6 cm� of the forearm for 8 hours. The results showed that a small percentage of dermally applied DEET was absorbed. The rate and amount of absorption were greater in the individuals who were treated with the 15 percent DEET solution. The level of radioactivity in the plasma declined rapidly after cessation of exposure. No human health effects data were documented from this study.[826]
A comparative study of dermal absorption on human tissue was conducted by the Health Canada Environmental Health Centre using three commonly used commercial insect repellent formulations. The three formulations — Off!�, Deep Woods�, and Muskol� — contained 14 percent, 24 percent, and 95 percent DEET, respectively. The total percentage of dermal absorption for the three formulations was 48 percent, 36 percent, and 17 percent for human skin, respectively. The study indicated that significant total cumulative quantities of DEET might be absorbed through the skin. Using the 17 percent absorption rate for Muskol�, 2.4 g of DEET would be absorbed by the 66-kg human volunteer who applied 15 ml of Muskol�. This represents an internal dose of 36 mg/kg. Two doses over an 8-hour workday would be a total dose of 72 mg/kg, which amounts to a total dose of 5.0 g for the average 70-kg person. The study indicated that none of the exposure levels presented a hazard to human health.[827]
Another study profiled the cumulative amount of DEET permeated across the skin from commercial mosquito repellents. DEET was found to continuously permeate (from the control and the four products containing DEET) through the human skin throughout a 36-hour period in significant amounts (0.5-1.5 mg/cm�). The cumulative amount of DEET permeated was proportional to the percentage of DEET found in the mosquito repellent. The study concluded that the permeation of DEET continues at high steady-state flux for 36 hours, and may continue to be released into the circulatory system hours after removal of the mosquito repellent from the skin surface. According to the study, none of the indicated levels of exposure presented a human health hazard.[828]
Reports of adverse events are rare, but most occur in children. The American Academy of Pediatrics (AAP) recommends application of only diluted DEET (<10 percent) to children. One study documented a survey of 71 poison control centers (PCCs) participating in the American Association of Poison Control Centers’ National Data Collection System for reports of adverse reactions to DEET between 1985 and 1989. The PCCs covered a wide area of the United States with a population of more than 180 million people. The survey indicated that there were 9,086 human exposures involving DEET-containing insect repellents reported to PCCs between 1985 and 1989. Of those exposed, 98.9 percent experienced either no effect or had short-lived symptoms involving mild irritation to the skin or mucous membranes. A total of 66 patients had symptoms classified as moderate (i.e., more pronounced or more prolonged than the minor effects), but all symptoms resolved. Five patients experienced more dramatic effects after being exposed to products containing 11-50 percent DEET. Two of the five patients experienced eye irritation, which was treated at home. One patient, a 17-year-old male who had saturated his clothing with 17.9 percent DEET, was ataxic and might have had a seizure. A 33-year-old male reported diminished sensation and hypotension 1 week after using a DEET product. Both individuals recovered fully. The study concluded that given the extensive use of DEET and lack of human clinical literature, the risk of adverse effects from the label-directed use of DEET-containing repellents is low.[829]
A 20-year-old soldier sought medical treatment when he experienced a persistent burning sensation and skin eruption after he had applied an insect repellent containing 33 percent DEET about 8 hours before the skin eruption had appeared. A diagnosis of irritant contact dermatitis was made based on the anamnestic and clinical data.[830]
DEET was detected in the blood of human volunteers within 2 hours of application on the volar surface of the forearm, and there was little evaporation from the skin. In one study, 48 percent of the dermally applied dose was absorbed within 6 hours.[831]
According to the EPA, since 1960 there have been 14 cases of seizure (including four deaths) potentially related to DEET exposure for which other more likely causes have not been identified. An additional 32 cases of seizure reported to the national DEET registry are currently under review. Thus, the final number of potential DEET-associated seizures since 1960 will fall between 14 and 46 cases. This range is subject to both over- and underreporting. Seizure coinciding with DEET use can be expected, given an estimated 15,000-20,000 afebrile (occurring without a fever) seizures in children ages 0-19 years estimated annually and an estimated 17 million children using DEET as often as 10 times a year. On the other hand, physicians may fail to check for history of DEET use or fail to report cases of seizure subsequent to DEET use. As noted in the Morbidity and Mortality Weekly Report editorial on the five cases in 1989, "Anecdotal reports of seizures are difficult to interpret. Taking all the cases together, it does appear that some of the cases are likely related to DEET toxicity, though it is not possible with certainty to say which ones."[832]
In 1989, the EPA notified the Centers for Disease Control and Prevention (CDC) and the Poison Control Center (PCC) through a physicians’ advisory of their concern for these health effects and asked them to report any new cases. The EPA also urged the manufacturer to undertake a review of PCC records. Neither the manufacturer’s review of more than 9,000 DEET exposures nor the EPA’s physicians’ advisory revealed any new cases of seizure that could be substantiated with medical records. Given only 14 - 32 cases since 1960 (the first case was reported in 1961) and 50 - 80 million people using DEET each year, the observed incidence of recognized seizures is about 1 per 100 million users.[833]
c. Inhalation Exposure
Human health effects studies providing toxicology benchmark dosages for this exposure route were not found in the research literature.
2. Subchronic Health Effects
a. Oral Exposure
Human health effects studies providing toxicology benchmark dosages for this exposure route were not found in the research literature.
b. Dermal Exposure
Human health effects studies providing toxicology benchmark dosages for this exposure route were not found in the research literature.
c. Inhalation Exposure
Human health effects studies providing toxicology benchmark dosages for this exposure route were not found in the research literature.
3. Chronic Health Effects
a. Oral Exposure
Exposure to DEET occurs in the short to intermediate term; chronic exposure is not expected.[834] Human health effects studies providing toxicology benchmark dosages for this exposure route were not found in the research literature.
b. Dermal Exposure
Data for estimating exposures from conventional consumer use practices are limited and insufficient. There are only a few reported estimates of exposure to occupational groups. Estimates of exposure over 6 months extrapolated from these limited data are: the upper 1 percent of the general population (estimated from a survey of 71 employees of 1 company) were exposed to a 15 percent concentration of DEET at a level of >1.65 g/dose for an exposure quantity of >214 g. Military personnel were exposed to a 75 percent concentration of DEET at a level of >8.25 g/dose for an exposure quantity of >1071 g. The upper 5 percent of Everglades Park Service employees surveyed were exposed to a 15 percent-75 percent concentration of DEET at a level of >2 kg/7 months (1 ml of a 75 percent formulation, 60/year) for an exposure quantity of >1710 g. For chronic dermal exposure, this indicates an exposure level of 136 mg/kg/d. National Park Service workers in the Florida Everglades, who rely heavily on DEET in summer months, have reported episodes of confusion and an abnormal sensation of decreased sweating while using DEET. Response to a neurobehavioral questionnaire by 143 of these workers indicated that there was a significant increase in the prevalence of certain neurological signs and symptoms, specifically muscle cramping, insomnia, irritability, and depression, among the workers who had an estimated dermal exposure to 4.25 g or more of DEET in an average week. Skin rash or blisters and difficulty in starting or stopping the urinary stream were significantly higher in this group of workers.[835]
Though the empirical testing does not demonstrate significant human toxicity to DEET, there are a few reports of individuals experiencing adverse effects after using a DEET product. In the past 35 years, 14 individuals reported suffering seizures after being exposed to DEET: 12 were children. Among these 14 incidents are 5 incidents that were reported to the New York State Department of Health in 1989. The EPA has analyzed these incidents and, at this time, cannot conclude that these seizures are or are not directly related to DEET exposure. In summary, the EPA concludes that, based on the currently available data, the use of DEET as an insect repellent does not pose a significant health risk to the US population in general for the following reasons: (1) DEET is not believed to be acutely toxic or carcinogenic, or significantly developmentally toxic or mutagenic at the doses tested; and (2) the available data do not support a direct link between exposure to DEET and reported seizure incidences (14 cases). The EPA concludes that DEET insect repellents will generally not cause unreasonable risks to humans or the environment.[836]
c. Inhalation Exposure
Because exposure to DEET occurs in the short to intermediate term, chronic exposure is not expected.[837] Human health effects studies providing toxicology benchmark dosages for this exposure route were not found in the research literature.
4. Risk Characterization: Comparison of HRA Modeled Dose Estimates to Non-Carcinogenic Health Effects
Please refer to the reference table(s) in Tab D, Section D.3.a.(2), "Other Human Benchmarks."
Application hazards for DEET were not modeled in the HRA Risk Characterization Table (Table 141) below so data are not compared.
Table 141. DEET, comparison of HRA doses to benchmarks (post-application exposure)
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
As indicated in the Table 141, no HRA route-specific dose estimates
are available for acute oral exposure for comparison with dosage data reported
in referenced literature.
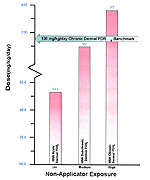
The literature cited for chronic dermal exposure is based on a concentration of DEET product ranging between 15-75 percent applied at >2 kg/7 months as compared with HRA dosage data that were based on concentrations of 75 percent and general application practices (Figure 17).
5. Uncertainty/Variability of this Comparative Risk Characterization
Because of the limited documented human dose response or occupational exposure/health effects data, there is significant uncertainty with this risk characterization. However, nearly a half-century of human DEET usage with minimal health consequences adds authenticity to the results of the comparison of HRA data and literature studies.
6. Risk Communication Summary
DEET is generally believed to be of low toxicity. Although the dermal absorption of DEET has been known since its introduction, its use has continued because it is believed that it does not present a dermatologic or toxic hazard to humans.[838]
Benchmark dosage data are lacking for all exposure levels and routes. Based on the published benchmarks at which human health effects occur, the HRA calculated dose estimates at the high chronic dermal dosage levels are within a range that can cause rare health effects in humans.
Figure 17: Representation of Estimated Risk
Comparative representation of published human health risk to the HRA route-specific dose estimates. The above graph represents the lowest concentration of DEET at which human health effects occur in the literature compared to the HRA estimated exposure of Gulf War servicemembers. The arrow represents human health effects data based on occupational exposure of 15-75% DEET concentration over a seven year period. This level can then be compared to the exposure route that had the highest reported HRA dose estimate for all exposure levels.
C. Permethrin
1. Acute Health Effects
a. Oral Exposure
In a reported suicide attempt, a 59-year-old male drank approximately 600 ml of 20 percent permethrin emulsion. Vomiting and diarrhea occurred after ingestion. On admission to the hospital, loss of consciousness and metabolic acidosis were observed. When he regained consciousness, the patient complained of a burning sensation in the oral cavity. He received fluid therapy after gastric lavage and recovered without severe complications. Apart from initially impaired consciousness, no clinical neurotoxicity occurred.[839] For acute oral exposure, this ingestion indicates an exposure level of 1623 mg/kg/d.
According to the Presidential Advisory Committee on Gulf War Veterans’ Illnesses, there are few reported cases of permethrin poisoning in humans, most likely because a large dose is required to cause poisoning. Humans rapidly detoxify and excrete permethrin. Clinical signs of immediate permethrin poisoning following large oral doses become evident within 2 hours and include incoordination, ataxia, hyperactivity, and convulsions, followed by prostration, paralysis, and/or death. The Committee found no reports of long-term effects from permethrin poisoning in humans.[840]
b. Dermal Exposure
A group of volunteers were given applications to an area of 4 cm� on the ear lobe of 0.05 ml of a field-strength preparation of technical (94-96 percent active ingredients) or formulated (32-36 percent) permethrin (0.13 mg/cm�) or of the inert ingredients. The intensity of paraesthesia induced by permethrin was fourfold stronger than that induced by a similar application of fenvalerate, permethrin being the least active compound for both the technical and formulated preparations. The inert ingredients elicited no cutaneous sensation. Paraesthesia appeared after a latent period of about 30 minutes, peaked between 8 and 12 hours, and disappeared after about 24 hours.[841]
One of 28 subjects with pediculosis pubis treated with a 1- percent permethrin rinse developed mild scrotal erythema and irritation 12 hours post-application. No dose estimate data were provided in the literature. Of 10 scabies patients treated with one application of 25 g (range, 21-32 g) of a 5 percent permethrin cream, followed by a thorough washing approximately 8-20 hours after treatment, six had limited, mild-to-moderate not pre-existing eczema on the scabies-affected skin at one or more examinations. For acute dermal exposure, this indicates an exposure level of 17.85 mg/kg/d. Permethrin has caused dermal irritation after topical exposure.[842]
Permethrin absorption from wearing treated (0.125 mg/cm�) military BDUs would be about 0.0006 mg/kg/d in humans. If the commercial 0.5 percent aerosol spray were used, human exposure would be markedly decreased, with the fabric treatment rate being reduced to about 0.025 mg/cm�. Though prelaundering treated fabrics removes up to 55 percent of the permethrin, it does not significantly alter the leaching rate during the first week of wear.[843]
The Extension Toxicology Network (EXTONET) indicates that permethrin is a moderately to slightly toxic pesticide in EPA Toxicity Class II or III, depending on the formulation. Formulations are placed in Class II because of their potential to cause eye and skin irritation.[844]
In a study by Farquhar, Hutchinson et al. (1981), permethrin exposure was performed on 10 individuals (4 men and 6 women). After applying 1- percent permethrin to the subjects, 30 percent developed skin irritation. In another study performed by Farquhar, Hutchinson et al., 10 male volunteers whose clothing had been impregnated with permethrin (3.8 mg/d) showed no signs of toxicity. A study by Le Quesne and Maxwell et al. (1980) that evaluated findings among 23 pest control workers occupationally exposed to multiple compounds, including permethrin. Although workers reported tingling, burning, and a rash starting at 30 minutes and lasting up to 8 hours postexposure, these findings were not exhibited among workers exposed to permethrin alone.[845]
After permethrin was introduced as an alternative to the treatment of head lice in humans, data were gathered on possible adverse effects from the use of a 1- percent permethrin creme rinse. Results reported from 18,950 individuals from 37 local public health departments showed few adverse reactions. The observed rate was approximately 2.2 adverse events per 1000 administrations.[846]
Pyrethroids, particularly permethrin and d-phenothrin, are safe and effective when used in recommended applications. Studies show that these compounds are potentially toxic at extremely high exposures; however, when used in conventional ways, only minor skin irritation in sensitive individuals’ results. Clinical manifestations subside after short periods when the inciting exposure is removed.[847]
c. Inhalation Exposure
Four of five workers in Sweden who packed conifer seedlings for 6 hours in a tunnel that had been sprayed 1 hour earlier with a 2 percent aqueous solution of permethrin, resulting in atmospheric concentrations of 0.011-0.085 mg/m� permethrin in the breathing zone, did not excrete detectable amounts of acid pyrethrin metabolites in the urine. One shorter individual whose face was close to the plants and who had the highest concentration of permethrin in the breathing zone excreted 0.26 �g/ml permethrin acid metabolites in the urine the following morning; in the afternoon, excretion was below the detection limit of the method. A group of five workers who planted the treated conifer seedlings were exposed to nondetectable- to low-permethrin levels in the breathing zone (mean, 2E-03 mg/m�; range, not detected — 6.0E-03 mg/m�) and excreted no detectable amount of permethrin metabolites in the urine.[848] For acute inhalation exposure, the lowest observed effect level based on the atmospheric concentration of 0.011 mg/m� was 9.4E-04 mg/kg/d. At the atmospheric concentration of 0.085 mg/m� the exposure level was 7.3E-03 mg/kg/d. No health effects were noted for either exposure level.
One known symptom from inhaled permethrin is cutaneous paresthesia described as burning, tingling, or stinging. This generally disappears within 1 day after removal of pyrethroids, but it persisted for more than 3 months in one case.[849] No dose estimate data were provided in the literature.
2. Subchronic Health Effects
a. Oral Exposure
Human health effects studies providing toxicology benchmark dosages for this exposure route were not found in the research literature.
b. Dermal Exposure
Human health effects studies providing toxicology benchmark dosages for this exposure route were not found in the research literature.
c. Inhalation Exposure
Human health effects studies providing toxicology benchmark dosages for this exposure route were not found in the research literature.
3. Chronic Health Effects
a. Oral Exposure
Human health effects studies providing toxicology benchmark dosages for this exposure route were not found in the research literature.
b. Dermal Exposure
A study was performed of 22 pest control operators of whom 3 were specifically exposed to permethrin. The subjects were exposed to a normal commercial application of pyrethroid mix containing permethrin for 1-21 years. No blood, heart, lung, liver, or central nervous system abnormalities were documented. There was no correlation between the number of complaints and pyrethroid metabolite concentration in urine. Only fatigue was more common in the pyrethroid-exposed group. No specific pyrethroids were discussed.[850] No dose estimate data were provided in the literature.
c. Inhalation Exposure
Human health effects studies providing toxicology benchmark dosages for this exposure route were not found in the research literature.
4. Risk Characterization: Comparison of HRA Modeled Dose Estimates to Noncarcinogenic Health Effects
Please refer to the reference table(s) in Tab D, Section D.3.a.(2), "Other Human Benchmarks."
Table 142. Permethrin, comparison of HRA doses to benchmarks (application exposure)
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Based on Table 142, the HRA route-specific dose estimates are below the range where human health effects were reported in the literature. For example, a comparison of doses in an acute dermal exposure scenario shows that the dose at which no human health effects were observed is higher than the HRA estimated dose. When comparing acute inhalation doses, the dose at which no human health effects occur is above the HRA estimated dose. One area of exception is the acute oral exposure data that have no HRA dose estimate data available for comparison (Figure 18).
Table 143. Permethrin, comparison of HRA doses to benchmarks (post-application exposure)
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
No literature data are available for non-applicator doses to compare with the HRA route-specific absorbed dermal dose estimates.
5. Uncertainty/Variability of this Comparative Risk Characterization
Because of limited documented human dose response or occupational exposure/health effects data, there is significant uncertainty with this risk characterization. Minimal human data identified from the literature means extrapolated comparisons of health effects data were necessary. In addition, there is uncertainty because of the limited data on identified human test groups. For these reasons, caution should be exercised when drawing conclusions based on these data.
6. Risk Communication Summary
Benchmark dose study data
are not available for all human health exposure levels and routes. The HRA calculated
dose estimates reflected in the applicator and non-applicator tables are below
doses that cause health effects in humans based on published benchmark data
found in the scientific literature and reported in this study.
Figure 18: Representation of Estimated Risk
Comparative representation of published human health risk to the HRA route-specific dose estimates. The above graph represents the lowest concentration of Permethrin at which human health effects occur in the literature compared to the HRA estimated exposure of Gulf War servicemembers. The arrow represents the most conservative NOEL for acute dermal exposure route from the human health effects studies. This level can then be compared to the exposure route that had the highest reported HRA dose estimate for all exposure levels.
D. d-PHENOTHRIN
1. Acute Health Effects
a. Oral Exposure
Human health effects studies providing toxicology benchmark dosages for this exposure route were not found in the research literature.
According to the World Health Organization (WHO), d-phenothrin has not been reported to cause human poisoning or other toxic effects in more than a decade of use. WHO concluded that d-phenothrin, when used as recommended, is not likely to pose a hazard to either human health or the environment. WHO also reported that d-phenothrin may be present in concentrations of up to 4 mg/kg in stored wheat, 0.8 mg/kg in flour, and 0.6 mg/kg in baked goods consumed by humans.[851]
b. Dermal Exposure
The material safety data sheets reviewed revealed mild human dermal hazard for d-phenothrin preparations.[852] Application of a 0.2 percent solution to head hair for 2 hours is a recommended standard treatment for head lice in the United Kingdom.[853] We speculate that a solution would not be recommended for treatment if it were known to cause health effects, especially in children, if it were inadvertently left in beyond the recommended time. Therefore, this treatment regimen represents an acute dermal no-observed-effect-level (NOEL) for humans. Assuming 1 ounce of shampoo would be applied to the head and skin for treatment of head lice, this would equate to approximately 56 mg on the skin (0.2 percent of 28 g). This would calculate to a potential dose rate for dermal contact of 0.8 mg/kg. Assuming a dermal absorption factor of 0.02 as stated in the Health Risk Assessment (HRA), this would calculate to about 0.016 mg/kg as an absorbed dermal NOEL for a 70-kg individual.
According to WHO, a 3-dose dermal application of 32-mg d-Phenothrin at 3-day intervals to human hair is a recommended treatment for lice. Using the calculations above this would amount to 96 mg on the human skin over 3 days, or a potential dermal dose of 1.3 mg/kg over the 3-day period (or 0.45 mg/kg/d [0.44-0.67 mg/kg/d reported]). No significant abnormalities, dermal irritation, clinical signs, or blood biochemical parameters were reported. The d-phenothrin was washed off 1 hour post-application and could not be detected in the blood at the minimum detection level of 6.0E-04 mg/kg.[854] An estimate of an acute absorbed dermal does of d-phenothrin would be 6.0E-03 mg/kg/d. The acute dermal absorbed range could then span a minimum of 6.0E-03 mg/kg/d to a possible maximum of 0.016 mg/kg/d for human body-lice treatment.
c. Inhalation Exposure
WHO reported that household aerosol spraying is not expected to create levels of exposure greater than 0.5 mg/m3 d-phenothrin. The initial concentration of d-phenothrin sprayed, how long this exposure persisted, and any indications of adverse health effects are unknown and were not reported. According to WHO, general human population exposure is expected to be very low, but precise data were lacking and values were not provided.[855]
Assuming a typical human inhalation exposure with no adverse effects is 0.5 mg/m3, an estimate of an acute human inhalation 24-hour NOEL is 0.17 mg/kg/d. A more conservative acute human inhalation NOEL is 7.1E-03 mg/kg assuming only 1 hour of exposure at 0.5 mg/m3 d-phenothrin.
The material safety data sheets reviewed indicated the acute inhalation hazards for d-phenothrin preparations were caused by other ingredients, propellants, and solvents rather than the active ingredient, d-phenothrin. The MSDSs made no reference of an occupational exposure limit (OEL) for d-phenothrin established by OSHA, NIOSH, ACGIH, or any other organization.[856] WHO indicated that with reasonable work practices, hygiene measures, and safety precautions, d-phenothrin is unlikely to pose an occupational health hazard.[857] No other acute inhalation human health effects studies were found in the research literature.
2. Subchronic Health Effects
a. Oral Exposure
Human health effects studies providing toxicology benchmark dosages for this exposure route were not found in the research literature.
b. Dermal Exposure
Human health effects studies providing toxicology benchmark dosages for this exposure route were not found in the research literature.
c. Inhalation Exposure
Human health effects studies
providing toxicology benchmark dosages for this exposure route were not found
in the research literature.
3. Chronic Health Effects
a. Oral Exposure
Human health effects studies providing toxicology benchmark dosages for this exposure route were not found in the research literature.
b. Dermal Exposure
Human health effects studies providing toxicology benchmark dosages for this exposure route were not found in the research literature.
c. Inhalation Exposure
Human health effects studies providing toxicology benchmark dosages for this exposure route were not found in the research literature.
4. Risk Characterization: Comparison of HRA Modeled Dose Estimates to Noncarcinogenic Health Effects
Please refer to the reference
table(s) in Tab D, Section D.3.a.(2), "Other Human Benchmarks."
Table 144. d-Phenothrin, comparison of HRA doses to benchmarks (application exposure)
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The acute dermal applicator exposure to d-phenothrin is estimated to be 1.04E-04 mg/kg/d in the HRA. A subchronic high d-phenothrin exposure is estimated at 4.15E-04 mg/kg/d in the HRA. Comparing this to the most conservative acute human dermal NOEL of 6.0E-03 mg/kg/d, we find the HRA dose estimate is 0.016 times a conservative standard dermal treatment for human lice eradication. Subchronic human dermal d-phenothrin data are unavailable, but the applicator exposure is estimated to be about 0.066 times the most conservative acute standard dermal treatment for lice eradication.
The acute inhalation applicator exposure to d-phenothrin is estimated at 3.55E-05 mg/kg/d - this is approximately 5.0E-03 times the estimated human inhalation NOEL of 7.1E-03 mg/kg/d. No subacute human NOEL was found in the literature, but the HRA estimation of 1.42E-04 mg/kg/d is 0.02 times the conservative estimated human inhalation NOEL of 7.1E-03 mg/kg/d (Figure 19.)
Table 145. d-Phenothrin, comparison of HRA
doses to benchmarks (post-application exposure)
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Dermal post-application exposure scenarios were not calculated because of the low level of exposure estimated. This is scientifically sound for non-applicator dermal scenarios for d-phenothrin exposure. Based on human dermal treatment regimens and high margins of safety for applicator exposures in relation to them, non-applicator dermal exposure would be far lower and less significant to any dermal human health effects range.
5. Uncertainty/Variability of Comparative Risk Characterization
Because of a lack of documented dose response or occupational exposure/health effects data - except for dermal lice treatment - reliable characterization of human health effects with HRA estimations for inhalation of d-phenothrin is uncertain. The most confident human NOEL comparison is with the human dermal experience because d-phenothrin is routinely prescribed as a topical treatment for lice and the concentration range is known.
Human usage experience, however, indicates little hazard or acute human health effects through oral or inhalation routes. Unfortunately, there is a lack of human health effects data and no good documentation in the environmental and occupational literature reviewed to date. The amount of residue present in foodstuffs and the expected acute inhalation exposure for typical use of d-phenothrin provides an uncertain benchmark of some tolerable short-term inhalation exposure, but assumptions had to be made that this was a NOEL. This assumption itself interjects uncertainty.
Counteracting this, the lack of human health effects data after more than a decade of worldwide usage indicates a high degree of safety with normal use. Although not discussed here, animal data of a variety of species tends to support this assumption as well. Data have not been found indicating human subchronic or chronic effects by any exposure route.
6. Risk Communication Summary
The actual, documented human health effects database for d-phenothrin is extremely sparse. Data reviewed about human health effects, when compared with calculated exposures of Gulf War veterans, seem to be in the range for acute exposures, with a large and lower exposure difference for both dermal and inhalation exposure. Longer-term, subchronic exposures are also well below human acute NOELs, although subchronic human health effects values for adequate and direct comparison with this exposure are not available.
Based on what has been
estimated and compared to date, evidence for acute and subchronic human health
effects from exposure to the pesticide d-phenothrin has not been presented for
any exposures of Gulf War veterans estimated in the HRA.
Figure 19: Representation of Estimated Risk
Comparative representation of published human health risk to the HRA route-specific dose estimates. The above graph represents the lowest concentration of d-Phenothrin at which human health effects occur in the literature compared to the HRA estimated exposure of Gulf War servicemembers. The arrow represents the most conservative NOEL for acute dermal exposure route from the human health effects studies. This level can then be compared to the exposure route that had the highest reported HRA dose estimate for all exposure levels.
E. AZAMETHIPHOS
1. Acute Health Effects
CIBA-GEIGY (now Novartis) has stated that human health effects from experiences specific to humans from mild to moderately severe poisoning include headache, weakness, sweating, indisposition, vomiting, nervousness, and difficulty swallowing. A severe exposure may result in profuse sweating, eye pain, miosis, muscular twitching, slurred speech, hypersalivation, respiratory distress, colic, heart complaints, convulsions, and unconsciousness. No dosages were reported for these human health effects.[858]
a. Oral Exposure
Human health effects studies providing toxicology benchmark dosages for this exposure route were not found in the research literature.
b. Dermal Exposure
According to CIBA-GEIGY, azamethiphos may cause sensitization through skin contact.[859] Health effects for acute dermal exposure may be similar to the health effects for acute oral exposure.
Human health effects studies providing toxicology benchmark dosages for this exposure route were not found in the research literature.
c. Inhalation Exposure
Health effects for acute inhalation exposure may be similar to the health effects for acute oral and dermal exposure.
Human health effects studies providing toxicology benchmark dosages for this exposure route were not found in the research literature.
2. Subchronic Health Effects
Although little exposure data are available for azamethiphos, subchronic exposure health effects for this cholinesterase (ChE) inhibitor may be similar to the effects of an acute exposure.
Human health effects studies
providing toxicology benchmark dosages for this exposure route were not found
in the research literature.
3. Chronic Health Effects
Few data are available on chronic exposure to azamethiphos. Health effects for a chronic exposure may be similar to the effects of an acute or subchronic exposure.
Human health effects studies providing toxicology benchmark dosages for chronic oral, dermal, and inhalation exposure routes were not found in the research literature.
4. Risk Characterization: Comparison of HRA Modeled Dose Estimates to Noncarcinogenic Health Effects
Please refer to the reference table(s) in Tab D, Section D.3.a.(2), "Other Human Benchmarks."
Because of a lack of dose
response data for human health effects from azamethiphos exposure, we could
not make a comparison between the Health Risk Assessment (HRA) doses and any
peer-reviewed studies.
Table 146. Azamethiphos, comparison of HRA doses to benchmarks (application exposure)
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Table 147. Azamethiphos, comparison of HRA doses to benchmarks (post-application
exposure)
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
5. Uncertainty/Variability of this Comparative Risk Characterization
Because of a lack of health effects data on human exposure to azamethiphos, we could not make a comparative risk evaluation.
6. Risk Communication Summary
Because of a lack of health effects data on human exposure to azamethiphos, caution should be exercised when using this pesticide until epidemiological and toxicological studies become available.
F. METHOMYL
1. Acute Health Effects
Methomyl is a carbamate pesticide initiating cholinesterase (ChE) inhibition. The acute toxic action of methomyl is characterized by signs of anticholinesterase action such as lacrimation, profuse salivation, tremor, and pupil constriction. Methomyl has a well-known mechanism of toxic action with particular toxicity by the acute oral and inhalation routes. As with other carbamate pesticide active ingredients, methomyl is excreted rapidly and does not accumulate in mammalian tissue. If exposure is suspended, ChE inhibition and its symptoms rapidly reverse.
a. Oral Exposure
Accidental and suicidal poisonings provide the bulk of human exposure data for methomyl. One instance of accidental poisoning comes from five Jamaican fishermen who added methomyl to their meal instead of salt. Methomyl had been stored in an unlabeled tin can and was mistakenly used in preparing "roti," an Indian dish. Only minutes passed before three of the men began twitching, trembling, and frothing at the mouth. All died within 3 hours of ingestion. Estimations from food samples show that the individuals consumed between 12 mg/kg and 15 mg/kg methomyl, which is considered a lethal dose.[860]
In nonfatal cases, illness generally lasts less than 24 hours. Based on qualitative and quantitative methomyl toxicity characteristics, 0.03 mg/kg/d is unlikely to cause adverse effects in humans by any exposure route. Accidental and intentional human poisoning data identify similar acute levels of methomyl toxicity in humans to that found in laboratory animals.[861]
The deaths of a 31-year old woman and her son concur with the previously cited lethal methomyl dose. Examination after their deaths identified 15.4 mg/kg methomyl concentrations. The estimated doses for the son and his mother were 13 mg/kg and 55 mg/kg, respectively.[862]
A 79-year-old man and his 73-year-old wife attempted double suicide by ingesting methomyl powder. The woman died 19 hours after ingestion despite intensive care. An autopsy revealed a large number of miliary hemorrhages found in both thalami of the brain. Her husband recovered after 10 days of treatment. Analysis revealed the methomyl concentration was 44 µg/g in the wife's serum sample collected 1 hour after ingestion, and 0.2 µg/g in the blood sample collected at autopsy. The methomyl concentration in the husband's blood sample collected 28 hours after ingestion was 0.01-0.1 µg/g. An estimated 11 g methomyl were ingested between the 2 of them. Methomyl powder, weighing approximately 5 g, amounts to approximately 30 mg/kg.[863]
b. Dermal Exposure
Because of the slower absorption phase of methomyl, acute dermal toxicity is low. Acute dermal exposures allow for recovery time from toxic action; therefore, the effect is never fully exerted.[864]
c. Inhalation Exposure
A health surveillance study in 22 healthy spraymen showed significant T-wave changes (including inversion) following a 5-day exposure to methomyl. Significant changes in plasma ChE and lactic dehydrogenase activities were also noticed. This is the first reported type of change in occupationally exposed subjects following exposure to a carbamate compound. Study results indicate that these changes are probably related directly to methomyl rather than its toxicity through ChE inhibition. The study identifies significance of these changes and the need for further investigation.[865]
In an unpublished document, more than 225 poisonings were reported between 1972 and 1973 following inhalation exposure to dry formulations of methomyl. Some exposure incidences resulted in serious acute reactions, but none were fatal. After this compound was reformulated as a liquid, cases decreased to fewer than 10 per year.[866]
In another case, methomyl was found in the blood of a pilot who died when his aircraft crashed while he was spraying methomyl. Circumstances of the pilot's case suggest that the methomyl poisoning probably occurred by inhalation and dermal absorption. The pesticide active ingredients in his blood were measured by gas chromatography with flame photometric detection and the results confirmed by mass spectrometry with direct liquid injection through a liquid chromatography interface. The whole blood methomyl concentration was 570 ng/ml.[867]
Specific dosage-related acute human inhalation studies were not found in the research literature. For this reason, data from the acute oral human studies will be used for making comparisons to the Health Risk Assessment (HRA) exposure estimates.
2. Subchronic Health Effects
a. Oral Exposure
Human health effects studies providing toxicology benchmark dosages for this exposure route were not found in the research literature.
b. Dermal Exposure
Human health effect studies providing toxicology benchmark dosages for this exposure route were not found in the research literature.
c. Inhalation Exposure
Human health effect studies providing toxicology benchmark dosages for this exposure route were not found in the research literature.
3. Chronic Health Effects
a. Oral Exposure
Human health effect studies providing toxicology benchmark dosages for this exposure route were not found in the research literature.
b. Dermal Exposure
Human health effect studies providing toxicology benchmark dosages for this exposure route were not found in the research literature.
c. Inhalation Exposure
One study identified chronic inhalation exposure in a plant manufacturing methomyl. The workers' average employment duration was two years. Eleven workers were hospitalized for illnesses related to methomyl exposures. The highest hospitalization rates occurred in areas involved with chemical packaging (27 percent), production (22 percent), and plant maintenance (9 percent). Clinical evaluation revealed 46 percent of the 11 packaging workers experienced the highest frequency of acute cholinergic symptoms, including miosis, blurred vision, nausea or vomiting, muscle weakness, fatigue, and increased salivation. No precise dose response or chronic health effects were determined and reliable ChE measurements were not made.[868]
Human health effects studies providing specific toxicology benchmark dosages for this exposure route were not found in the research literature.
4. Risk Characterization: Comparison of HRA Modeled Dose Estimates to Noncarcinogenic Health Effects
Please refer to the reference
table(s) in Tab D, Section D.3.a.(2), "Other Human Benchmarks."
Table 148. Methomyl, comparison of HRA doses to benchmarks (application exposure)
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The HRA/Literature comparison table for application exposure identifies HRA calculated route-specific doses below those identified in the literature. If equivalent absorption is assumed for both oral and inhalation routes, the HRA estimated dose for acute inhalation of methomyl is approximately 2.0E-03 times the level where health effects were observed in the literature. Differences in dermal exposure levels are less significant (Figure 20).
Table 149. Methomyl, comparison of HRA doses to benchmarks (post-application exposure)
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
A similar pattern appears in the post-application exposure HRA/Literature table. Data from the HRA enable comparisons with the chronic inhalation, subchronic oral, and chronic oral routes. The greatest estimated dose of 3.47E-02 mg/kg/d listed under chronic inhalation is slightly above the literature-extrapolated dose at which human health effects occurred.
5. Uncertainty/Variability of this Comparative Risk Characterization
Because of a limited amount of documented human dose response and occupational exposure/health effects data, there is significant uncertainty with this risk characterization. Minimal human data identified from the literature necessitate extrapolated comparisons of subchronic and chronic health effects from acute doses and assumptions of equivalent route-specific absorption. In addition, there is uncertainty because of limited data about identified human test groups.
For these reasons, caution should be exercised when drawing conclusions from these data.
6. Risk Communication Summary
Based on limited published
human health benchmarks for methomyl, the HRA calculated dose estimates seem
to fall below doses where identified human health effects occur. It should be
noted, however, that this is based on the assumptions communicated throughout
this text and a statistically significant comparison is not possible because
of a lack of sufficient dose response data.
Figure 20: Representation of Estimated Risk
Comparative representation of published human health risk to the HRA route-specific dose estimates. The above graph represents the lowest concentration of Methomyl at which human health effects occur in the literature compared to the HRA estimated exposure of Gulf War servicemembers. The arrow represents the most conservative LOEL for acute oral exposure route from the human health effects studies. This level can then be compared to the exposure route that had the highest reported HRA dose estimate for all exposure levels.
G. DICHLORVOS
1. Acute Health Effects
a. Oral Exposure
Health effects from ingesting dichlorvos may include pallor, nausea, vomiting, diarrhea, abdominal cramps, headache, dizziness, eye pain, blurred vision, constriction or dilation of the eye pupils, tearing, salivation, sweating, and confusion. These symptoms can occur from within a few minutes to 12 hours after the initial exposure. Severe poisoning affects the central nervous system by producing slurred speech, reflex loss, weakness, fatigue, involuntary muscle contractions, twitching, tremors of the tongue or eyelids, and eventually paralysis of arms, legs and respiratory muscles. Loss of bowel and bladder control, psychosis, irregular heartbeats, unconsciousness, convulsions, coma, and death through respiratory failure or cardiac arrest may also occur with severe poisoning.[869] No benchmark dosages are reported for the above symptoms.
b. Dermal Exposure
Acute exposure through skin contact may cause localized sweating and involuntary muscle contractions within 15 minutes to 2 hours after exposure.[870] Acute health effects from dermal contact with dichlorvos include the same health effects as those observed in oral exposure, with symptoms occurring a few minutes to several days after the initial contact.[871] No dermal benchmark dosages were reported for the above symptoms.
c. Inhalation Exposure
Acute health effects from inhalation of dichlorvos include the same as those observed in oral and dermal exposure. Inhaling dichlorvos may also produce a feeling of tightness in the chest, wheezing due to bronchospasm, runny nose, and frontal headache.[872]
According to a 1972 report, a research team subjected a group of human volunteers to a single exposure of dichlorvos 1.0 mg/m3 for 7.5-8.5 hours. The plasma cholinesterase levels in these individuals dropped 20-25 percent.[873] Assuming that the dose was administered to a 70-kg individual over an 8-hour period, the acute human LOEL would be 0.114 mg/kg/d.
d. Multiple Exposure Routes
A 1984 investigation reported by the US Environmental Protection Agency describes the effects of dichlorvos on applicators and residents following fumigation of houses. The applicators were exposed to average levels of 0.21 mg/m3 for approximately 25.5 minutes. Assuming that the dose was administered to a 70-kg individual over a 25.5-minute period, the acute human LOEL would be 1.2E-03 mg/kg/d. The residents of the fumigated homes were exposed to an average concentration of 0.21 mg/m3 for about 15.8 hours. Based on these data, the dose for a 70-kg individual would be 0.047 mg/kg/d. Data on two of the dichlorvos applicators showed decreases in plasma cholinesterase activity of 21 percent and 59 percent, respectively. The mean plasma cholinesterase activity for the residents a day after the application of dichlorvos was reduced only slightly (-7.9 percent) when compared with cholinesterase levels before the pesticide application. The only clinical effect noted in the residents was headache.[874]
2. Subchronic Health Effects
a. Oral Exposure
In a 1967 study cited by the EPA, groups of five males were fed 1.0, 1.5, 2.0, or 2.5 mg doses of dichlorvos once a day for 28 days. Plasma cholinesterase activity was 71 percent of controls at the end of the 28 days in the group receiving 2.0 mg/d and was 70 percent of controls at the end of 20 days in the group administered 2.5 mg/d. There was no significant effect on erythrocyte cholinesterase activity, and there were no clinical symptoms of exposure in the subjects.[875]
In another EPA-cited study,
107 male volunteers were administered oral doses of dichlorvos ranging from
0.1 mg/kg to 16.0 mg/kg. Another group of 44 men received placebo pellets. Clinical
symptoms reported by some of the treated and control groups were stomach rumbling,
nausea, and diarrhea. Laboratory tests showed a decrease in cholinesterase activity
in the treated subjects, and the decrease in this activity became greater as
the dose increased. Erythrocyte cholinesterase activity also decreased with
increasing dichlorvos administration, but the decrease was less than that observed
in the plasma. The experiment had to be terminated in most subjects after a
week due to the dramatic rate of decrease in plasma and erythrocyte cholinesterase
activity from the daily dosings. An attempt to gradually increase the dose in
the subjects produced similar cholinesterase depression; at 16 mg/kg, the experiment
was terminated after only 5½ days.[876]
b. Dermal Exposure
Human health effects studies providing toxicology benchmark dosages for this exposure route were not found in the research literature.
c. Inhalation Exposure
The EPA reports a limited occupational study in which six formulators and reactor workers in a pesticide manufacturing plant were exposed to dichlorvos for 20 days at an average air concentration of 0.1 ppm. The research team in this study observed a decrease in plasma cholinesterase activity and Vitamin A levels in 5 workers, noting that these levels were recovering slowly after a 13-day period of nonexposure.[877]
However, in another study, volunteers were exposed to dichlorvos concentrations ranging from 0.14 mg/m3 to 0.33 mg/m3 for 30 minutes every hour for 10 hours. This study was performed over 14 consecutive days. At the end of the 2-week period, the researchers found no change in cholinesterase activity in the volunteers.[878]
3. Chronic Health Effects
In most aspects, health effects may be the same for chronic dichlorvos as those for acute exposure for each exposure route. More specific chronic exposure effects may include impaired memory and concentration, disorientation, severe depression, irritability, confusion, headache, speech difficulties, delayed reaction times, nightmares, sleepwalking, and drowsiness or insomnia. Flu-like symptoms may also occur.[879]
Human health effects studies providing toxicology benchmark dosages for the chronic oral, dermal, and inhalation exposure routes were not found in the research literature.
4. Risk Characterization: Comparison of HRA Modeled Dose Estimates to Noncarcinogenic Health Effects
Please refer to the reference table(s) in Tab D, Section D.3.a.(2), "Other Human Benchmarks."
Because there are no Health
Risk Assessment (HRA) doses reported for a scenario in which applicators are
exposed to dichlorvos, a comparison of HRA modeled doses to literature-cited
benchmark health effect levels cannot be made. A LOEL value for an acute exposure
to dichlorvos was calculated from the research performed by Gold et al. (1984).[880]
The primary route of exposure was probably inhalation in this study,
but dermal and oral exposure could also have occurred.
Table 150. Dichlorvos, comparison of HRA doses to benchmarks (post-application exposure)
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
In the post-application exposure scenarios, the HRA estimated doses were very similar to the levels at which health effects occurred in the research literature. At low exposures, the HRA estimated dose is only 0.25 times the benchmark dose for inhalation exposure. At the medium level exposure, the HRA dose and the benchmark dose are nearly equal, while at the high level exposure the HRA estimated dose is slightly above the benchmark inhalation dose. It should be noted again that the HRA doses were compared with a NOEL from a subchronic inhalation study (Figure 21).
5. Uncertainty/Variability of this Comparative Risk Characterization
The accuracy of the comparison of HRA doses to benchmark doses in the post-application exposure study is uncertain. The subchronic NOEL (inhalation) dose is higher than the acute LOEL (multiple route) dose. These data were extrapolated from two separate studies that had small test groups.
6. Risk Communication Summary
Based on the published benchmarks at which human health effects occur used in this study, the HRA calculated dose estimates seem to be very close to the doses that cause health effects in humans. However, because this study encompasses only a small amount of benchmark doses for all exposure levels and routes from human health studies, caution should be exercised when comparing human health effects to HRA modeled exposure estimates for this compound.
Figure 21: Representation of Estimated Risk
Comparative representation of published human health risk to the HRA route-specific dose estimates. The above graph represents the lowest concentration of Dichlorvos at which human health effects occur in the literature compared to the HRA estimated exposure of Gulf War servicemembers. The arrow represents the most conservative NOEL for all inhalation exposures route from the human health effects studies. This level can then be compared to the exposure route that had the highest reported HRA dose estimate for all exposure levels.