H. CHLORPYRIFOS (3,5,6-TRICHLORO-2-PYRIDINYL)
1. Acute Health Effects
a. Oral Exposure
According to the Agency for Toxic Substances and Disease Registry, "short-term oral exposure (one day) to low (milligrams) levels of chlorpyrifos causes dizziness, runny nose, confusion, salivation, and rapid heart rate. Short-term oral exposure to moderate (grams) levels of chlorpyrifos may cause paralysis, seizures, loss of consciousness, and death. Reports … also show that short-term exposure to chlorpyrifos may cause muscle weakness weeks after the original symptoms have disappeared. This is typical of organophosphate-induced delayed neuropathy (OPIDN). In humans, few cases of true OPIDN have been observed. However, true OPIDN has been seen in laboratory animals following very high doses of the pesticide. No permanent effects of short-term chlorpyrifos exposure in people have been found."[881]
Two human studies were conducted to determine specific dosages at which human health effects begin to occur. In the first study, by Coulston et al. (1972), male volunteers (4/dose group) were given daily oral doses of 0, 0.014, 0.03, or 0.1 mg chlorpyrifos/kg/d. The initial research protocol proposed that the doses be given for 7 weeks, 9 days, 21 days, and 28 days, respectively. It was reported on Day 9, however, that individuals of the 0.1-mg/kg group reached plasma cholinesterase (ChE) inhibition levels of 36 percent-82 percent and one individual complained of blurred vision, runny nose, and faintness. The report concluded that no significant plasma ChE inhibition was observed in the 0.03-mg/kg group after 21 days or in any of the other test groups. Plasma ChE levels returned to normal 25 days postexposure.[882]
Human no-observed-effect-levels (NOELs) and lowest-observed-effect-levels (LOELs) for repeated doses were derived from this study and published based on human data.[883] For plasma ChE inhibition, the NOEL is 0.03 mg/kg/d and the LOEL is 0.1 mg/kg/d. For red blood cell (RBC) acetylcholinesterase (AChE), the NOEL is 0.1 mg/kg/d, but no LOEL could be derived based on available data. There is uncertainty from this study because of the small test population (4 men/dose group) and the wide range of variability observed in the plasma cholinesterase inhibition (36 percent-82 percent in the 0.1-mg/kg/d group). In addition, the investigators attributed the health effects observed in the one individual to common cold, but the US Environmental Protection Agency’s Hazard Identification Assessment Review Committee (HIARC) concluded that a cold could not have caused the blurred vision.[884]
The second human study, conducted by Nolan et al. (1984), was performed using six Caucasian male volunteers ages 27-50 years. Each volunteer was given a single oral dose of 0.5 mg/kg in pellet form.[885] The volunteers were subsequently monitored for 30 days to determine plasma ChE and RBC AChE inhibition as well as the chlorpyrifos primary metabolite 3,5,6-trichloro-2-pyridinol (3,5,6-TCP) concentrations in the blood and urine. At no point during the 30 days were any clinical symptoms of toxicity or RBC AChE inhibition observed. Although the original paper reported no RBC AChE inhibition, a later report on the study by the EPA noted that peak AChE inhibition of 11 percent-52 percent was reached on Day 4.[886] This conclusion seems to be based on a graph presented in the original paper, but the EPA provides no reasoning for the conclusion. To remain consistent with published data, the conclusions made by Nolan et al. (1984) are used in this review. The maximum mean plasma cholinesterase inhibition observed was 15� 1 percent compared with baseline levels. Based on 3,5,6-TCP data, it was estimated that 72� 11 percent of the oral dose was absorbed.
This study, in conjunction with the study mentioned above, led to publication of human NOEL and LOEL data for single-dose exposures.[887] For plasma ChE inhibition, the NOEL is 0.1 mg/kg and the LOEL is 0.5 mg/kg. For RBC AChE, the NOEL is 0.5 mg/kg. This study is subject to the same uncertainties discussed previously, such as a small test population and variability of observed ChE inhibition among volunteers. This will become increasingly apparent when analyzing the dermal absorption study cited below, which was conducted using the same six volunteers.
Based on the observation of Hayes et al. (1980) that clinical manifestations of acute human toxicity occur when RBC AChE is inhibited at 50 percent of the baseline, an acute oral dose greater that 0.5 mg/kg would be required for adverse health effects.[888] Unfortunately, there is a lack of data to statistically support this observation. Most reports of chlorpyrifos-induced human toxicity lack dosage data, exposure route information, or discussion of potential contributing factors. Another question arises when referring to the neurological effects reportedly caused by chlorpyrifos and other organophosphate pesticide active ingredients. Investigators in the two human studies did not test for neuropsychological effects, and in cases where clinical tests were run the doses and exposure routes are not available.
OPIDN is associated with exposure to a few specific organophosphates, possibly including chlorpyrifos at very high doses. This syndrome is caused by the inhibition of neurotoxicity target esterase (NTE) in neural tissue, which triggers the degradation in sensory and motor axons of the peripheral nerves and/or spinal chord.[889] The inhibition of NTE occurs within hours of intoxication, and OPIDN has only been reported in cases where the dose was extremely high.[890]
In one reported case, a 42-year old male ingested ~300mg/kg chlorpyrifos in an attempted suicide.[891] Plasma cholinesterase activity measured 36 hours postexposure was nearly zero and the individual remained comatose with cholinergic symptoms for 17 days. Day 30 measurements of RBC and plasma cholinesterase as well as NTE were all significantly below normal levels, but began to rise from that point on. "On Day 43, the patient complained of weakness and paresthesias in the legs and symmetrical reduction of tendon reflexes was noted. Sensory conduction velocity was reduced and signs of denervation of some muscles [were] recorded. On Day 62, the leg weakness was more severe, the patient showed some gait impairment, and the tendon reflexes were absent. On electomyograph, the muscles of the legs showed symmetrical signs of denervation with signs of spontaneous activity." Further examination showed reduction of motor and sensory conduction velocities as well as signs of axon and myelin degeneration. Other cases of attempted suicide and accidental chlorpyrifos exposures with similar symptoms have been reported, but definitive doses are not provided.[892]
b. Dermal Exposure
Nolan et al. (1984) conducted a human dermal absorption study in connection with the oral study mentioned above, using the same six volunteers.[893] One received two 0.5 mg/kg dermal doses, one dissolved in methylene chloride and the other in dipropylene glycol methyl ether [DPGME], and the remaining five received doses of 5.0 mg/kg dissolved in DPGME. It was determined that 1.35� 1 percent of the dermal dose was absorbed based on blood chlorpyrifos measurements following exposure. The mean plasma ChE inhibition in the five volunteers was 13 percent, but further analysis showed a large discrepancy in levels between individuals. A 20 percent decrease was observed in one individual; there was no decrease in levels for two of the volunteers and a high variability in levels for the other two volunteers.
Based on the information from this study, if one were to assume that a maximum of 2.35 percent of the 5.0 mg/kg dermal dose was absorbed, that would give an absorbed dose of approximately 0.12 mg/kg. This 0.12 mg/kg dose could be the assumed absorbed human acute dermal LOEL even though two of the individuals showed no plasma ChE inhibition at this dose.
c. Inhalation Exposure
Fenske et al. (1990) performed measurements to determine the maximum air concentration of chlorpyrifos following residential broadcast applications.[894] Samples were taken from both ventilated and nonventilated rooms. Wipe samples taken within 0.5 hours following application were 1.69 �g/cm2 and 3.90 �g/cm2 in the ventilated and nonventilated rooms, respectively. Chlorpyrifos vapors measured 100 cm above the carpet never exceeded 20 �g/m3 in ventilated rooms, and maximum levels greater than 60 �g/m3 were measured in nonventilated rooms.
A poison control center report on chlorpyrifos exposures over a 10-year period provides four fatalities potentially caused by inhalation of high levels of chlorpyrifos.[895] Two separate exposures occurred in adult males while spraying chlorpyrifos under houses. Both experienced cardiopulmonary arrest that resulted in their deaths. No other causal factors are suggested and no dose estimates were provided. For these and other reasons, it is difficult to make conclusions with respect to these cases.
A third exposure occurred when, "an 85-year-old male was admitted to the hospital for treatment of pneumonia and sepsis." He had symptoms of lethargy, diarrhea, and fever. Four days before he was hospitalized, his home had been treated with a chlorpyrifos containing compound. One-week post application, soil samples taken from under the house contained 560 ppm chlorpyrifos and the air-conditioning filter contained 46 PPM When the house was swabbed, chlorpyrifos levels were measured at 170 �g/ft2. His vital signs upon hospital admission were: heart rate 100 beats per minute; blood pressure 122/50 mmHg; temperature 99.6�F; and "rales were ausculated over the lungs." The patient died 13 days after being admitted to the hospital. Caution should be exercised when drawing conclusions from this case study because of the lack of a definitive dose, the age of the victim, and uncertainty of exposure route.
The fourth fatality occurred when a 39-year-old female inhaled an entire aerosol can containing chlorpyrifos, hydrocarbons, and pyrethrins. Her symptoms included cardiac arrest en route to the hospital, multiple seizures, neuromuscular abnormalities, and excessive pulmonary secretions. She died 16 days postexposure. Among the difficulties in drawing conclusions from this case study are lack of dose information, concentration of chlorpyrifos, and the presence of hydrocarbons and pyrethrins in the insecticide mixture.
Because of a lack of dose response data in humans for acute inhalation exposure, we will assume for the purpose of this review that equivalent absorption would occur in both oral and inhalation routes, and use the acute oral LOEL of 0.1 mg/kg/d for Health Risk Assessment (HRA) comparison.
2. Subchronic Health Effects
a. Oral Exposure
Two human studies were conducted to determine specific dosages where human health effects begin to occur. In the first study, male volunteers (4/dose group) were given daily oral doses of 0, 0.014, 0.03, or 0.1 mg chlorpyrifos/kg/d.[896] The initial research protocol proposed that the doses be given for 7 weeks, 9 days, 21 days, and 28 days, respectively. However, on Day 9, individuals of the 0.1-mg/kg group reached plasma ChE inhibition levels of 36-82 percent and one individual complained of blurred vision, runny nose, and faintness. The report concluded that no significant plasma ChE inhibition was observed in the 0.03-mg/kg group after 21 days or in any of the other test groups. Plasma ChE levels returned to normal 25 days postexposure.
From this study, human Noels and LOELs for repeated doses were derived and published.[897] For plasma ChE inhibition, the NOEL is 0.03 mg/kg/d and the LOEL is 0.1 mg/kg/d. For RBC AChE, the NOEL is 0.1 mg/kg/d, but no LOEL could be derived based on the available data. There is uncertainty in this study because of the small test population (4 men/dose group) and the wide range of variability observed in the plasma cholinesterase inhibition (36-82 percent in the 0.1-mg/kg/d group). In addition, the investigators attributed the health effects observed in the one individual to a common cold, but the HIARC concluded that a cold could not have caused the blurred vision.[898]
b. Dermal Exposure
Human health effects studies providing toxicology benchmark dosages for this exposure route were not found in the research literature. For this reason, the data from the acute dermal human studies will be used to make subchronic comparisons to the HRA exposure estimates.
c. Inhalation Exposure
Because of a lack of dose response data for subchronic inhalation exposure, we will assume for the purpose of this review that equivalent absorption would occur in both oral and inhalation routes and use the subchronic oral NOEL of 0.03 mg/kg/d for HRA comparison.
3. Chronic Health Effects
a. Oral Exposure
For the purpose of this report, it will be assumed that the subchronic data collected from the human study reported above will remain constant and will be used in the HRA comparison.
b. Dermal Exposure
Human health effects studies providing toxicology benchmark dosages for this exposure route were not found in the research literature. For this reason, the data from the acute dermal human studies will be used for making chronic comparisons to the HRA exposure estimates.
c. Inhalation Exposure
Because of a lack of dose response data for chronic inhalation exposure, we will assume for the purpose of this review that equivalent absorption would occur in both oral and inhalation routes and use the subchronic oral NOEL of 0.03 mg/kg/d for HRA comparison.
4. Risk Characterization: Comparison of HRA-Modeled Dose Estimates to Non-Carcinogenic Health Effects
Please refer to the reference table(s) in Tab D, Section D.3.a.(2), "Other Human Benchmarks."
Table 151. Chlorpyrifos, comparison of HRA doses to benchmarks (application exposure)
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
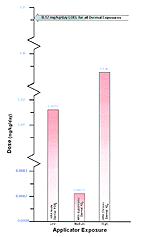
Based on the HRA/literature comparison table for application exposure, the HRA calculated route-specific doses seem to be well below the doses at which human health effects occur in the literature. For example, assuming equivalent absorption in both oral and inhalation routes, the HRA-estimated dose for acute inhalation of 45 percent liquid chlorpyrifos is approximately 1.8E-05 times the dose at which health effects would occur. In this same group, the HRA PDRD is 5.3E-05 times the dermal dose required to see human health effects (Figure 22).
This same pattern holds true for the post-application exposure scenarios compared in the table below. At the highest estimated dose of 2.28E-02 listed under high chronic ADD, the literature-extrapolated dose at which human health effects occur is 4 times greater than the estimated dose. This is true only if we assume that subchronic and chronic exposures would result in the same human health effects observed following acute exposure. Based on these assumptions, all estimated doses seem to be well below the level at which human health effects have been reported in the literature.
Table 152. Chlorpyrifos, comparison of HRA doses to benchmarks (post-application exposure)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
5. Uncertainty/Variability of this Comparative Risk Characterization
Several human studies have been found in the literature involving more than one individual over multiple dose ranges to document human health effects from chlorpyrifos exposure. This reduces uncertainty of the dose response relationship for human health effects data regarding this compound. However, variability of human response was also documented in the literature cited. The certainty and confidence regarding chlorpyrifos human health effects benchmarks in this review is increased because they have been used by EPA and FDA as the basis to establish more conservative chronic oral reference doses.[899] , [900]
6. Risk Communication Summary
Based on published benchmarks,
the HRA calculated dose estimates seem to be well below doses where human health
effects occur. It should be noted, however, that this is based on the assumptions
communicated throughout this text and a statistically significant comparison
is not possible because of a lack of sufficient dose response data.
Figure 22: Representation of Estimated Risk
Comparative representation of published human health risk to the HRA route-specific dose estimates. The above graph represents the lowest concentration of Chlorpyrifos at which human health effects occur in the literature compared to the HRA estimated exposure of Gulf War servicemembers. The arrow represents the most conservative LOEL for all dermal exposures from the human health effects studies. This level can then be compared to the exposure route that had the highest reported HRA dose estimate for all exposure levels.
I. DIAZINON
1. Acute Health Effects
a. Oral Exposure
The US Agency for Toxic Substances and Disease Registry (ATSDR) reports a case of a 54-year-old female who died after ingesting an estimated 293 mg/kg dose of diazinon.[901] The woman died of petechial hemorrhages in the brain, stomach, and gastric tract. A research paper, citing a National Institute for Occupational Safety and Health (NIOSH) source from 1977, documented a lowest possible lethal human dosage of diazinon at 50 mg/kg, but the source of this value was not stated.[902]
Five individuals who ingested a range of 240-916 mg/kg diazinon suffered health effects but recovered.[903] Data indicate that death in humans can occur at 293 mg/kg and above, and possibly as low as 50 mg/kg. The 916-mg/kg level also represents an upper level for humans at which significant health effects occur, with recovery with prompt medical treatment.
An acute human dose response lowest-observed-effect-level (LOEL) for red blood cell (RBC) acetylcholinesterase (AChE) from exposure to diazinon has not been found in the literature. RBC AChE inhibition is considered a clinical marker of organophosphate exposure, including diazinon. Human plasma AChE inhibition will fluctuate more rapidly than RBC changes and will regenerate quickly after exposure to organophosphate pesticide active ingredients ceases.
Inhibition of 50 percent of human RBC AChE from normal levels is a level where symptoms of acute toxicity will appear.[904] Another source states that a 50 percent level of RBC AChE inhibition will present severe human cholinergic poisoning symptoms.[905] A scientific literature source has not been located documenting a 50 percent RBC inhibition to a human dosage of diazinon.
In a series of tests involving 2-4 human volunteers, a dose of 0.05 mg/kg/d diazinon was administered orally for up 28 days. In this study a 35-40 percent plasma cholinesterase (ChE) inhibition was presented; no effect of RBC cholinergic activity was found and no other adverse health effects were reported.[906] The range of 0.025-0.05 mg/kg/d can be considered a range where human plasma ChE suppression occurs, human RBC cholinergic activity does not, and no other health effects are reported.
In a study on human male volunteers, three individuals were dosed with 0.02 mg/kg/d and 0.025 mg/kg/d diazinon in cornstarch capsules for 38 and 43 days, respectively. Plasma and RBC ChE inhibition was assessed every 2-3 days. All three volunteers in the 0.025-mg/kg/d group showed consistent inhibition between 8 percent and 38 percent for plasma AChE relative to pretest values, and two of the three volunteers in the 0.02-mg/kg/d group showed consistent inhibition between 9 percent and 38 percent for plasma AChE. Volunteers did not present RBC AChE inhibition at either dosage, however. This study (as reported for a period of 1-7 days) concludes that the acute oral LOEL and no-observed-effect-level (NOEL) for plasma AChE inhibition in humans are 0.025 mg/kg/d and 0.02 mg/kg/d, respectively.[907]
b. Dermal Exposure
Dosage-related acute dermal studies of diazinon in humans have not been found in the research literature; however, there are reports of diazinon health effects from dermal contact. ATSDR reported that no studies were available that indicate that human deaths were related to dermal exposure to diazinon. Other scientific reports indicate that some humans have died from dermal contact with diazinon, so lethality by dermal contact, though unlikely, should be considered a potential health effect for humans. There are reports that diazinon caused allergic skin irritations from contact with human skin, but a dosage was not reported.[908]
ATSDR reported a study in which human volunteers were found to have a 3 percent-4 percent dermal absorption after 4-24 hours of a solution applied to the forearm and abdomen in acetone or lanolin grease.[909] If 4 percent diazinon is the accepted human absorption rate, the applied dermal dose to human skin is 25 times the absorbed dermal dose to produce similar toxic effects as those described by the oral route. In the absence of human dermal dose response studies, the suite of dosage-related human symptoms for acute, subchronic, and chronic oral toxicity for diazinon are assumed to be the same for dermal contact.
c. Inhalation Exposure
High inhalation exposure is expected to present AChE inhibition health effects in humans. In combination with dermal exposure, these health effects can include neurological symptoms leading to respiratory failure and cardiac arrest.[910] However, a dosage level in humans strictly from high inhalation exposure has not been found from literature sources reviewed so far.
Diethylthiophosphate (DETP), a diazinon metabolite, was found in the urinary samples of pesticide applicators.[911] A study of 99 applicators and supervisory non-applicator personnel measured exposure levels of 1.5 mg and 0.02 mg whole-body diazinon respectively per shift and showed an increase in DETP. Pre-shift measurements were carried out at the beginning of the study and at 39 days. Hence, the values present human effects information in an acute and initial subchronic range of exposure.
The study examined 18 physical frank health effects that included tiredness, dizziness, headache, muscle twitching, weakness in the hands and legs, tingling in the toes and fingers, depression, nausea, and tightness of chest. An entire battery of neurobehavioral tests was performed. Some postshift differences were observed in symbol digit speed and memory accuracy. The authors concluded that diazinon exposure was not responsible for the small performance shift observed in postshift symbol digit speed and memory accuracy because the performance deficit was not related to dose response. They further concluded that no evidence was found of adverse dose response related behavioral effects (including DETP level increase) among pest control workers who had low-level exposure to diazinon for relatively short durations.
Calculating an upper, lower, and mean dose range for applicators (which constitutes the higher exposed group) based on a 70-kg individual we arrive at 1.4E-03-0.15 mg/kg/d (0.1-10.4 mg/d exposure), with a median of 0.02 mg/kg/d (1.5 mg/d exposure). The median exposure is at the human oral NOEL, the upper bound measured exposure is approximately 7 times the NOEL with no indication of adverse health effects. The dose for post-application exposure ranged from 2.8E-05-2.8E-03 mg/kg/d (2.0E-03-0.2 mg/d exposure), with a median of 2.8E-04 mg/kg/d (0.02 mg/d exposure). Applicators wore personal protective equipment (PPE) ranging from gloves to respirators, so this exposure is not a measure of absorbed dose. The non-applicators did not wear PPE and these data are a measure of absorbed dose.
Given the scope of physical and neuropsychological tests, the number of subjects involved, and the fact that the study was based on diazinon alone and actual exposure was assessed, this study provides highly confident reassurance that the human NOEL established - and exposures below it - are absent physical and psychological effects for acute and subchronic time frames, including expression of a biomarker of exposure (DETP). Although this study had inhalation measurements, the whole-body diazinon exposure techniques that were used examined all dermal, oral and inhalation exposure routes. Therefore, it will be used for all three exposure routes.
Inhalation is assumed to be a combination of aerosol and vapor diazinon, particularly in application of the insecticide. High concentrations of diazinon capable of producing toxic effects purely from vapor inhalation under normal circumstances are not likely.
In a North Carolina study, air concentrations of diazinon in commercial pesticide buildings, service vehicles, and food-preparation areas following pesticide application were measured in ambient air. Storage and office rooms had a mean 284 ng/m3 air concentration of diazinon with a range of 85-837 ng/m3. Storage and office areas had the highest diazinon concentration. Another study found a maximum of 3.4 µg/m3 for a 14-hour period in a retail garden store that sold diazinon. Airborne levels of 38 µg/m3 diazinon were found in rooms 21 days after a crack-and-crevice application. Airborne diazinon levels peaked at 167 µg/m3 and 27 µg/m3 4 hours post-application. The airborne concentrations in this case indicated that building occupants should not enter treated rooms that are unventilated until 2 days post-application.[912]
A quick, calculated 24-hour dose would indicate a 2.9E-05 mg/kg/d dosage for the highest ambient sample (837 ng/m3), 0.013 mg/kg/d (38 µg/m3) in the 21-day crack-and-crevice treatment measurement, and a maximum dosage of 0.05 mg/kg/d if the maximum concentration of 163 µg/m3 4 hours after a broadcast spray application of diazinon remained constant over a day. The last example would be the only one where the human lowest observed adverse effect level (LOAEL) of 0.02 mg/kg/d would be exceeded.
The National Institute for Occupational Safety and Health (NIOSH) established a Recommended Exposure Level (REL) of 0.1 mg/m3 for diazinon for a 10-hour work shift, considered a safe working level, or NOAEL, of inhalation exposure for an average individual. Assuming inhalation of 1 m3 air/hour - and, thus, a dose of 1.0 mg/d divided by a 70-kg individual-we end up with an acute level (a single working day NOAEL) of 0.014 mg/kg/d for diazinon via the inhalation route in humans. This is well within a factor of 2 for the NOEL discussed for oral toxicity reported above and considered equivalent for toxicological comparison. The absorbed dose of diazinon by the inhalation route is considered to be 100 percent and the health effects ranges are considered equivalent to those listed for oral toxicity.
2. Subchronic Health Effects
a. Oral Exposure
A subchronic human dose-response LOEL for RBC AChE from exposure to diazinon has not been found in the literature. RBC AChE inhibition is considered a clinical marker of organophosphate exposure, including diazinon. Human plasma AChE inhibition will fluctuate more rapidly than RBC changes and will regenerate quickly after exposure to organophosphate pesticide active ingredients ceases.
Inhibition of 50 percent of human RBC AChE from normal levels is a point at which symptoms of acute toxicity will appear.[913] According to another source, a 50 percent RBC AChE inhibition level will result in severe human cholinergic poisoning symptoms.[914] No resource documenting a 50 percent RBC inhibition in humans has been found in the research literature.
In a series of tests involving 2-4 human volunteers, a dose of 0.05 mg/kg/d diazinon was administered orally for up 28 days. In this study, a 35-40 percent plasma ChE inhibition was presented, no effect of RBC cholinergic activity was found, but no other adverse health effects were reported.[915] A range of 0.020-0.05 mg/kg/d will be considered a subchronic oral dosage range at which human plasma ChE suppression occurs, human RBC cholinergic activity does not, and no other health effects are reported.
In a study on human male volunteers, three subjects received diazinon doses of 0.02 mg/kg/d and 0.025 mg/kg/d in cornstarch capsules for 38 and 43 days, respectively. This study, as applied to a three months-to-lifetime period, concluded that the subchronic oral LOEL for plasma AChE inhibition in humans is 0.02 mg/kg/d.[916] This LOEL is 0.005 mg lower than that in the acute study. No subchronic NOEL was stated for plasma AChE inhibition.
b. Dermal Exposure
Human health effects studies providing toxicology benchmark dosages for this exposure route were not found in the research literature.
c. Inhalation Exposure
Subchronic studies describing human health effects strictly from diazinon inhalation were not found. The multiple-route diazinon metabolite study of pesticide applicators described in the acute inhalation exposure section above includes table values for subchronic toxicity, since the study was conducted over a 39-day period. The description of dose response conclusions is the same as in this previous description and are not repeated here.[917]
3. Chronic Health Effects
a. Oral Exposure
An adequate chronic dose response study of human health effects of diazinon from the oral exposure route, either toxicological or epidemiological in nature, is unavailable.[918]
A chronic oral LOEL is reported in the literature, which is supported by human data. In a study on human male volunteers, three individuals received diazinon doses of 0.02 mg/kg/d and 0.025mg/kg/d in cornstarch capsules for 38 and 43 days, respectively. This study, as applied to a three months-to-lifetime period, concluded that the chronic oral LOEL for plasma AChE inhibition in humans is 0.02 mg/kg/d.[919] This LOEL is 0.005 mg lower than in the acute study. No NOEL was stated for plasma AChE inhibition.
b. Dermal Exposure
Human health effects studies providing toxicology benchmark dosages for this exposure route were not found in the research literature.
According to the World Health Organization (WHO), no cases of chronic symptoms of delayed neurotoxicity in humans, even after diazinon poisoning by acute means, have been reported. The animal data, however, would indicate otherwise.[920]
c. Inhalation Exposure
Chronic dose response studies of human health effects of diazinon from the inhalation exposure route, either toxicological or epidemiological in nature, are unavailable.[921]
4. Risk Characterization: Comparison of HRA Modeled Dose Estimates to Non-Carcinogenic Health Effects
Please refer to the reference
table(s) in Tab D, Section D.3.a.(2), "Other Human Benchmarks."
Table 153. Diazinon, comparison of HRA doses to benchmarks (application exposure)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The high exposure estimated in the Health Risk Assessment (HRA) dermal applicator scenario assumed daily application of a large amount of pesticide (40 gallons) and no use of personal protective equipment. This scenario estimated an absorbed dermal exposure of 0.191 mg/kg/d and 0.198 mg/kg/d for handwand and backpack application. This exposure is about 10 times above the documented NOEL in humans for any physiological change. This value is 3 times the upper bound human LOEL of 0.05 mg/kg/d at which plasma AChE - but no RBC AChE inhibition and other frank health effects - has been documented in humans. It is not known at this time if this amount would cause significant (50 percent or more) RBC inhibition and lead to other cholinergic poisoning health effects.
If the medium or most likely diazinon applicator dermal exposure level for backpack power sprayers is examined, where more exposure is modeled to occur and where gloves and body dress are required, the highest modeled exposure is 2.9E-03 mg/kg/d. This is 0.015 times the NOEL of 0.02 mg/kg/d and 0.012 times the human LOEL of 0.025 mg/kg/d. Dermal exposures for hand sprayers are about 2.6E-03 times the 0.02 mg/kg/d human NOEL and 2.0E-03 times the 0.035 mg/kg/d human LOEL. The lowest modeled dermal diazinon application exposure scenarios are nearly 0.1 times the medium, putting the absorbed dermal diazinon dose at 1.6E-03 to far lower 1.0E-03 times the human NOEL and LOELs.
All inhalation diazinon exposures for applicators in the high (backpack sprayer) scenario are between 0.07 to 0.05 times the NOEL and LOEL respectively. The medium modeled inhalation exposures for applicators were 5.0E-04 times the NOEL, and the lowest modeled applicator exposures were more than 5.0E-05 times the NOEL for diazinon in humans (Figure 23).
Table 154. Diazinon, comparison of HRA doses to benchmarks (post-application exposure)
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The HRA estimated post-application high scenario (10 percent or less of field-deployed troops) modeled absorbed dermal doses of 0.0607 mg/kg/d diazinon, which is roughly 3 times the human NOEL of 0.02 mg/kg/d. This value is slightly above-and nearly equivalent with-the upper bound 0.05 mg/kg/d range at which human plasma AChE inhibition is observed from diazinon exposure but RBC and other health effects in humans are not.
5. Uncertainty/Variability of Comparative Risk Characterization
The LOEL/NOEL delta was only 0.005 mg/kg/d; therefore, one might expect greater sensitivity in some individuals, justifying a higher level of uncertainty in the NOEL, though not the LOEL. This uncertainty in the NOEL is further justified in that two of three subjects presented evidence of some plasma AChE inhibition at the lower dose, but not at the higher one.[922] This trend reverses the expected dose response curve.
However, greater certainty and less human variability are expected regarding the NOEL for human RBC AChE inhibition. At three low dosage levels, no human RBC AChE inhibition was presented in any test subjects at 0.02, 0.025, or 0.05 mg/kg/d diazinon-dosed groups. This is a fairly well-supported indictor in our review of the evidence (with three studies involving human volunteers, with three dosage levels) of the absence of RBC AChE inhibition as a health effect in humans at or below oral dosages of 0.05 mg/kg/d for acute and subchronic exposures and at or below a 0.02 mg/kg/d level for oral chronic exposure levels.
There are frank health effects due to cholinergic poisoning beyond AChE inhibition in humans. Qualitatively and quantitatively based on the human studies available, the acute oral levels of human health effects are the most certain. Oral human studies represent the data set with the largest number of dose response related studies.
Other benchmark levels of human health effects are less certain or do not exist. Because of this, oral studies were compared to the dermal and inhalation exposure routes. ATSDR concluded that scientifically supportive human data - primarily the oral route - exist for acute and subchronic (intermediate) exposures. Some of the human studies cited were uncertain because multiple routes of exposure or combinations of insecticides were involved. ATSDR noted in its review that there were no chronic toxicological and epidemiological studies in humans.[923] Therefore, the oral acute route provides the most certain characterized human health effects. Low-level diazinon exposure documenting the lack of health effects and plasma AChE inhibition is the best health effect characterization supported by human data. Other cholinergic toxic human symptoms are less supported by valid human dose response data and scientific literature.
For our characterization here, the absorbed dermal and inhalation doses are considered equivalent in toxic effects to the oral dosages described for humans. Based on the literature reviewed so far, below 0.05 mg/kg/d diazinon there is some confidence that the only human health effect is plasma AChE inhibition - there is no RBC AChE inhibition and no human health effects, behavioral or physiological, for acute and some subchronic time frames (e.g., 1 month).
6. Risk Communication Summary
a. Application Exposure Scenarios
For the modeled high application exposure scenarios, if diazinon exposures are in the range the HRA estimated, an exposure is possible that could cause suppression of the AChE plasma biomarker in humans. Other health effects are not comparable from human data at this time, and it is not certain that this exposure would significantly inhibit RBC AChE and, thus, give rise to other cholinergic health effects. It is also indicated in the HRA that this type of a high diazinon application scenario is rare because DoD prescribes PPE for this job.
All other diazinon application scenarios in the HRA, encompassing the medium and low ranges for the dermal and inhalation routes, are well below the human-based NOEL and LOEL. These scenarios are considered likely because DoD pesticide application protocols require PPE. Health effects would not be expected for troops that applied diazinon under these conditions.
b. Post-Application Exposure Scenarios
The largest numbers of troops were exposed passively to diazinon in the mess hall or latrine (the post-application scenario). If the HRA model is correct, the high post-application scenario would represent an area where human plasma AChE inhibition might be observed. This level is barely above the range at which there are human data to indicate absence of RBC AChE or other human health effects.
Medium and low absorbed
dermal and inhalation post-application doses of diazinon are not modeled.
Figure 23: Representation of Estimated Risk
Comparative representation of published human health risk to the HRA route-specific dose estimates. The above graph represents the lowest concentration of Diazinon at which human health effects occur in the literature compared to the HRA estimated exposure of Gulf War servicemembers. The arrows represent an acute NOEL, a subchronic LOEL, and a chronic NOEL dermal exposures from the human health effects studies. This level can then be compared to the exposure route that had the highest reported HRA dose estimate for all exposure levels.
J. MALATHION
1. Acute Health Effects
a. Oral Exposure
In humans, the symptoms of an acute oral exposure to this cholinesterase-inhibiting compound include nausea, abdominal cramps, sweating, blurred vision, numbness, tingling sensations in the extremities, motor incoordination, headache, dizziness, tremor, difficulty breathing or respiratory depression, and slow heartbeat. Severe poisoning may result in unconsciousness, loss of bowel and bladder control, convulsions, and/or death. The health effects depend on the gender and amount of protein in the diet of an individual.[924] The lowest published lethal oral dose for a man is 471 mg/kg, while the lethal oral dose for a woman is about half that amount at 246 mg/kg.[925]
In one reported case of acute oral poisoning, a 24-year-old male was found deceased with two partially filled bottles of a malathion-containing mixture. A day or two before his death, the man suffered from severe diarrhea and vomiting. The official cause of death was ruled myocardial failure due to acute malathion poisoning. The blood, urine, bile, gastric, and intestinal contents of the deceased were tested for malathion content. Malathion was not detected in the blood or urine samples; however, it was present in the bile at a concentration of 570 mg/L, in the gastric contents at 201 g/kg, and in the intestinal contents at 98 g/kg. The contents of the bottles found near the deceased were tested for malathion. An exact concentration was not issued, but one bottle contained 11 percent malathion and the other contained 54 percent malathion.[926]
b. Dermal Exposure
Human health effects studies providing toxicology benchmark dosages for this exposure route were not found in the research literature.
c. Inhalation Exposure
Human health effects studies providing toxicology benchmark dosages for this exposure route were not found in the research literature.
2. Subchronic Health Effects
a. Oral Exposure
According to the US Environmental Protection Agency's Integrated Risk Information System (IRIS) Database, 5 healthy male volunteers, ranging in age from 23 to 63 years, participated in a study by Moeller and Rider (1962). The volunteers were given malathion in gelatin capsules at three dose levels: 8 mg/d for 32 days, 16 mg/d for 47 days, and 24 mg/d for 56 days. Cholinesterase (ChE) activity was measured in the subjects twice weekly before, during, and after administration of this pesticide. The intermediate dose was determined to be a no-observed-effect-level (NOEL) of 0.23 mg/kg/d. The high dose caused a depression in plasma and red blood cell (RBC) ChE activity, although no clinically manifested side effects were observed. This dose, the lowest-effect-level (LEL), was 0.34 mg/kg/d.[927]
In another study, a group of human volunteers were fed very low doses of malathion for a month-and-a-half. During this period and afterwards, there were no significant effects on RBC ChE activity for this group. The exact dosage given in this study was not reported.[928]
b. Dermal Exposure
Human health effects studies providing toxicology benchmark dosages for this exposure route were not found in the research literature.
c. Inhalation Exposure
Human health effects studies providing toxicology benchmark dosages for this exposure route were not found in the research literature.
3. Chronic Health Effects
a. Oral Exposure
Human health effects studies providing toxicology benchmark dosages for this exposure route were not found in the research literature.
b. Dermal Exposure
Human health effects studies providing toxicology benchmark dosages for this exposure route were not found in the research literature.
c. Inhalation Exposure
Human health effects studies providing toxicology benchmark dosages for this exposure route were not found in the research literature.
4. Risk Characterization: Comparison of HRA Modeled Dose Estimates to Non-Carcinogenic
Health Effects
Please refer to the reference table(s) in Tab D, Section D.3.a.(2), "Other Human Benchmarks."
Table 155. Malathion, comparison of HRA doses
to benchmarks (application exposure)
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Based on the Health Risk Assessment (HRA)/literature comparison table for application exposure shown above, the HRA calculated route-specific doses are well below the doses correlated with human health effects that appear in the research literature. The exception is at high exposure levels with dermal absorption. At this level the HRA estimates range from 2 to 10 times the benchmark. An example of the HRA dose being well below the research literature value occurs when comparing the HRA estimated dose (1.79E-05 mg/kg/d) for subchronic inhalation of vapor from 57 percent liquid malathion (handwand application) to the level at which health effects occurred in the study by Moeller and Rider (1962).[929] The HRA dose is nearly 5.3E-05 times the dose at which health effects were observed. In the high exposure with dermal absorption scenario, the literature value and the HRA dose are nearly equal. In these comparisons, the absorption characteristics were assumed to be the same in the oral, dermal, and inhalation exposure routes (Figure 24).
The HRA estimated doses
for post-application exposure scenarios (see table below) are also well below
the levels at which health effects would be observed. The HRA subchronic dose
at a medium exposure level is about 0.01 times the dose that caused health effects
in the research literature, and at high exposure it is about 0.02 times the
observed dose. Again, it should be noted that these comparisons were made using
subchronic oral doses and not inhalation doses.
Table 156. Malathion, comparison of HRA doses to benchmarks (post-application exposure)
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
5. Uncertainty/Variability of Comparative Risk Characterization
Because of a lack of reportable dose response data, there is significant uncertainty with this risk characterization. Data for dermal and inhalation scenarios were not available and an assumption was made that the literature values for acute and subchronic oral exposure would have the same values for inhalation exposure as long as the absorption rate was the same. In addition, there is uncertainty because of limited data on identified human test groups.
6. Risk Communication Summary
Based on the published
benchmarks on human health effects used in this study, the HRA calculated dose
estimates seem to be well below doses that cause health effects in humans. However,
because of a lack of benchmark doses for all exposure levels and routes, caution
should be exercised when determining comparing human health effects to HRA estimated
dosages for this compound.
Figure 24: Representation of Estimated Risk
Comparative representation of published human health risk to the HRA route-specific dose estimates. The above graph represents the lowest concentration of Malathion at which human health effects occur in the literature compared to the HRA estimated exposure of Gulf War servicemembers. The arrow represents the most conservative NOEL for subchronic oral exposure route from the human health effects studies. This level can then be compared to the exposure route that had the highest reported HRA dose estimate for all exposure levels.