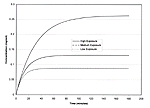
Figure 13 shows time plots of dichlorvos concentrations in the tent for the first 3 hours following the installation of the resin strip. Concentrations start at zero and rapidly approach the corresponding equilibrium concentrations. Table 53 presents a summary of the calculation of estimated concentrations.
Figure 13. Time plots of resin strip (dichlorvos) air concentrations inside tent
Table 53. Calculation of indoor air concentrations for dichlorvos, 20% resin strip
|
Exposure Scenario |
High |
Medium |
Low |
| Emission Rate (mg/h/strip) | 14.9 | 14.9 | 14.9 |
| Pest Strip Application (strips/1,000 ft3) | 1 | 1 | 1 |
| Emission Rate (mg/h/ft3) | 0.0149 | 0.0149 | 0.0149 |
| Emission Rate (mg/h/m3) | 0.526 | 0.526 | 0.526 |
| Air exchange rate (air changes per hour) | 2 | 4 | 6 |
| Half-life (h) | 13.6 | 13.6 | 13.6 |
| Decay constant (h-1) | 0.051 | 0.051 | 0.051 |
| Equilibrium concentration (mg/m3): | |||
| With Decay Included | 0.2566 | 0.1299 | 0.0870 |
| Without Decay | 0.2631 | 0.1315 | 0.0877 |
|
Average Concentration for 16 hours: |
|||
| With Decay Included | 0.2487 | 0.1279 | 0.0861 |
| Without Decay | 0.2549 | 0.1295 | 0.0868 |
Although the EPA recommends the use of the MCCEM in conducting high-end exposure assessments, investigators used the simple box model for the same reasons discussed under permethrin.
In the OPP report, [264] four different methods were used to estimate residential inhalation exposure to dichlorvos from pest strips. Three of these methods were based on dichlorvos air concentrations measured in homes using pest strips in a 1973 study.[265] The fourth method was based on a calculated dichlorvos emission rate (same one used in this analysis) and the use of a computerized EPA indoor air model, the MCCEM. The EPA’s fourth method is most similar to the approach used in this analysis. The MCCEM model calculates indoor air concentrations in various rooms within a structure, based on assumed fresh air infiltration rates and air migration patterns between rooms. MCCEM performs calculations similar to the box model, except that it allows numerous rooms (boxes) to be evaluated and can evaluate concentrations at different time intervals with different emission rates (although the EPA held the emission rate constant in its scenarios).
The EPA’s highest estimated dichlorvos exposure using the MCCEM method was 0.079 mg/kg/d for adult males over a 56-day exposure period. This daily dose is equivalent to an average air concentration of 0.43 mg/m3, based on a body weight of 70 kg, 15 h/d spent indoors and an inhalation rate of 12.9 m3/d while indoors. The house modeled by the EPA had an intermediate air exchange rate (3.57 air changes per hour) and a lower application rate (one pest strip per 1,900 ft3) compared with the scenarios modeled here. The air concentrations investigators estimated for GP tents, 0.09-0.26 mg/m3, are similar to the EPA’s estimate.
The 1973 indoor air measurement study used as the basis for three of the EPA’s exposure estimation methods showed lower dichlorvos air concentrations than those estimated with the models discussed above.[266] The highest measured dichlorvos concentration was 0.11 mg/m3, measured on the first day after installation of the strips. This is comparable to the concentrations estimated here for the low and medium exposure scenarios. The estimated concentrations for the high exposure scenario are somewhat higher than those measured. However, the usage rate of resin strips reported by the EPA (an average of one strip per 1,833 ft3 and a range of between one strip per 720 ft3/strip and one strip per 6,790 ft3) is generally smaller than the rate of one strip per 1,000 ft3 investigators assumed in the modeling analysis presented here.
Investigators did not use the 1973 measurement study as the basis of these exposure estimates for the following reasons:
For these reasons, investigators expected the 1973 measurement results would underestimate actual concentrations somewhat for the present exposure assessment.
d. Dichlorvos Doses - Post Application
Table 54 presents doses potentially resulting from post-application exposure to dichlorvos, 20% resin strip. The only dose listed, and the only one of concern, is the potential dose rate for inhalation (PDRI). Investigators did not calculate lifetime average daily doses (LADDs) because OPP does not currently provide a carcinogenic slope factor for dichlorvos by any exposure route (Tab D, Section D, "Toxicity Assessment").
Table 54. Dichlorvos, dose rates - post application for evaluation of noncarcinogenic effectsa
|
Formulation |
Exposure Group |
Exposure Point |
ABS |
PDRD (mg/kg/d) |
ADD (mg/kg/d) |
PDRI (mg/kg/d) |
| Dichlorvos, 20% resin strip | Low | - | - | - | - | 6.01E-03 |
| Medium | - | - | - | - | 3.32E-02 | |
| High | - | - | - | - | 9.62E-02 | |
|
||||||
a) |
PDRI = potential dose
rate for inhalation. A dash (-) indicates that the item is not applicable. BW = body weight. IRA = inhalation rate. ET = exposure time (mess and latrine). |
7. Emulsifiable Concentrates Used for Spraying
a. Application Scenarios
(1) Common Elements
Servicemembers applied the four emulsifiable concentrates to control pests such as: flies, mosquitoes, ticks, and/or fleas. They applied different ECs for different pests. Investigators evaluated application by low-pressure 2-gallon handwand sprayer and by backpack sprayer, as applicators used both methods. Application took place both outdoors and indoors. Outdoor applications were broadcast treatments typically made to the near-ground portions of buildings and tents, as well as to garbage containers, animal carcasses, and other places where the target pests congregated. Indoor applications would normally have been crack and crevice type treatments of locations such as mess and latrine facilities. In the course of such treatments the applicator directs a stream or cone of spray at cracks and crevices, such as commonly occur where a wall meets a floor. Investigators assumed applicators treated surfaces adjacent to the cracks, and that overspray could have been substantial. Additionally, building size, condition, and configuration impacted the relative fraction of surface area treated. Investigators assumed that applicators treated a much greater fraction of surface area in a small latrine as opposed to a large rectangular mess hall. For this assessment, rigid structures serve as the models. Applicators probably treated some tent interiors, but investigators did not specifically address these treatments, as they have no reason to believe that the exposures would be any higher. EC use by service is presented in Section IV.C, "Pesticides - Identification, Use, Exposures, and Potential Health Effects."
Investigators employed the following clothing scenarios based on review of military guidance and the PM exposure interviews:
The low- and medium-exposure levels represent normal and proper use of ECs, while the high-exposure level includes limited misuse (e.g., inadequate PPE).
As implied previously, one cannot take the percentile values listed for servicemembers wearing PPE on Table 13 at face value. For ECs, the percentile values presented would seem to indicate that 73-100% of servicemembers wore "adequate" PPE; however, much uncertainty surrounds these values: 1) adequate PPE was not defined in the interviews; 2) there is a low response rate for ECs (no higher than 64%); and 3) the data do not reflect the many qualitative statements provided by interviewees indicating that some applicators had little or no PPE.
Table 55 presents the common assumptions for application of ECs. The unit exposure values presented are from the PHED Guide. All values used to calculate the doses to applicators are presented in the following subsections. The equations used to calculate two of the values presented are as follows (refer to the following subsections for details):
VF =
where,
VF = volume of formulation used daily[268]
VS = volume of spray prepared daily
FC = finished concentration
WD = density of water
CS = concentration of active ingredient in formulationWA = CS x VF where,
WA = weight of active ingredient handled daily
All other equations used in dose calculations are provided in the associated tables in the following subsections.
Table 55. ECs, common assumptions for application
| Factor | Units | Definition/Explanation |
Assumptions by Level |
Source/Rationale | ||
|
Low |
Medium |
High |
||||
| UE | mg/lb a.i. | Unit dermal exposure | 0.43 | 0.43 | 100 | 1998 PHED Guide: handwand sprayer[269] |
| UE | mg/lb a.i. | Unit dermal exposure | 2.5 | 2.5 | 104a | 1998 PHED Guide: backpack sprayer[270] |
| UIE | mg/lb a.i. | Unit inhalation exposure | 0.003 | 0.003 | 0.03 | 1998 PHED Guide: handwand sprayer[271] |
| UIE | mg/lb a.i. | Unit inhalation exposure | 0.003 | 0.003 | 0.03 | 1998 PHED Guide: backpack sprayer[272] |
| WD | lb/gal | Density of water | 8.34 | 8.34 | 8.34 | TIM 24[273] |
a) |
"No glove" hand data were not provided in Scenario 34 from the PHED Guide; back calculation is not appropriate. Used "no glove" hand data from Scenario 32. |
(2) Chlorpyrifos, 45% Liquid (EC)
Twenty-five percent of the PM exposure interviews cited use of chlorpyrifos, 45% liquid (EC) (Table 13). Note that "chlorpyrifos, 45% liquid (EC)" as used throughout this health risk assessment means the 42% and/or 45% formulations; the difference being negligible in this assessment. Chlorpyrifos EC was used to control filth flies, sand flies, and mosquitoes, predominantly outdoors. Chlorpyrifos EC reportedly had some use indoors (cited by 8% of PM exposure interviews), in structures such as mess and latrines. TIM 24 lists both indoor and outdoor uses for chlorpyrifos EC, although it specifies outdoor use only for biting flies.[274] Table 56 presents the formulation-specific assumptions used for the application exposure assessment of chlorpyrifos EC.
Table 56. Chlorpyrifos, 45% liquid (EC) assumptions for applicationa
| Factor | Units | Definition/Explanation |
Assumptions by Level |
Source/Rationale | ||
|
Low |
Medium |
High |
||||
| EP | - | Exposure point | Outdoor | Outdoor | Indoor | TIM24;[275] PM interviews |
| VS | gal/d | Volume of spray prepared daily | 1 | 5 | 40 | PM interviews; OPP/HED[276] |
| FC | % a.i. | Finished concentration | 0.5 | 0.5 | 0.5 | TIM 24[277] |
| CS | lb/gal | Concentration of active ingredient in formulation | 4 | 4 | 4 | DowElanco[278] |
| VF | gal/d | Volume of formulation used daily | 0.010 | 0.052 | 0.42 | Equation in text. |
| WA | lb a.i. /d | Weight of a.i. handled daily | 0.042 | 0.21 | 1.7 | Equation in text. |
| ET | h/d | Exposure time | 1 | 5 | 12 | PM interviews |
| EF | d/mo | Exposure frequency | 1 | 13 | 30 | PM interviews |
| ED | mo | Exposure duration | 1 | 4 | 8 | PM interviews |
| ABS | - | Dermal absorption factor | 0.03 | 0.03 | 0.03 | ATSDR[279] |
a) |
A dash (-) indicates that the item is not applicable. |
(3) Diazinon, 48% Liquid (EC)
Fifteen percent of the PM exposure interviews cited use of diazinon, 48% liquid (EC) (Table 13). Diazinon EC was used to control ticks and fleas, predominantly outdoors. Diazinon EC reportedly had some use indoors (cited by 13% of PM exposure interviews), in structures such as mess and latrines. TIM 24 lists both indoor and outdoor uses for diazinon EC.[280] Table 57 presents the formulation-specific assumptions used for the application exposure assessment of diazinon EC.
Table 57. Diazinon, 48% liquid (EC) assumptions for applicationa
| Factor | Units | Definition/Explanation |
Assumptions by Level |
Source/Rationale | ||
|
Low |
Medium |
High |
||||
|
EP |
- | Exposure point | Outdoor | Outdoor | Indoor | TIM24;[281] PM interviews |
|
VS |
gal/d | Volume of spray prepared daily | 1 | 5 | 40 | PM interviews; OPP/HED[282] |
|
FC |
% a.i. | Finished concentration | 0.2 | 0.5 | 1.0 | TIM 24[283] |
|
CS |
lb/gal | Concentration of active ingredient in formulation | 4 | 4 | 4 | Octagon[284] |
|
VF |
gal/d | Volume of formulation used daily | 0.0042 | 0.052 | 0.83 | Equation in text. |
|
WA |
lb a.i./d | Weight of a.i. handled daily | 0.017 | 0.21 | 3.3 | Equation in text. |
|
ET |
h/d | Exposure time | 1 | 4 | 11 | PM interviews |
|
EF |
d/mo | Exposure frequency | 1 | 11 | 30 | PM interviews |
|
ED |
mo | Exposure duration | 1 | 4 | 9 | PM interviews |
|
ABS |
- | Dermal absorption factor | 0.04 | 0.04 | 0.04 | ATSDR[285] |
a) |
A dash (-) indicates that the item is not applicable. |
(4) Malathion, 57% Liquid (EC)
Seventeen percent of the PM exposure interviews cited use of malathion, 57% liquid EC (Table 13). Malathion EC was used to control filth flies, sand flies, mosquitoes, and ticks, predominantly outdoors. Malathion EC was reportedly used indoors as well (cited by 17% of PM exposure interviews), in structures such as mess and latrines. TIM 24 lists outdoor uses, but no relevant indoor uses, for malathion EC.[286] The label does mention use inside homes.[287] Thus, while indoor use of malathion may not have constituted misuse, it was not encouraged by military guidance. Table 58 presents the formulation-specific assumptions used for the application exposure assessment of malathion EC.
Table 58. Malathion, 57% liquid (EC) assumptions for applicationa
| Factor | Units | Definition/Explanation |
Assumptions by Level |
Source/Rationale | ||
|
Low |
Medium |
High |
||||
|
EP |
- | Exposure point | Outdoor | Outdoor | Indoor | TIM24;[288] PM interviews |
|
VS |
gal/d | Volume of spray prepared daily | 1 | 5 | 30 | PM interviews; OPP Health Effects Division[289] |
|
FC |
% a.i. | Finished concentration | 1.0 | 1.0 | 2.0 | TIM 24[290] |
|
CS |
lb/gal | Concentration of active ingredient in formulation | 5 | 5 | 5 | Octagon[291] |
|
VF |
gal/d | Volume of formulation used daily | 0.017 | 0.083 | 1.0 | Equation in text. |
|
WA |
lb a.i. /d | Weight of a.i. handled daily | 0.083 | 0.42 | 5.0 | Equation in text. |
|
ET |
h/d | Exposure time | 1 | 3 | 6 | PM interviews |
|
EF |
d/mo | Exposure frequency | 1 | 10 | 30 | PM interviews |
|
ED |
mo | Exposure duration | 1 | 5 | 8 | PM interviews |
|
ABS |
- | Dermal absorption factor | 0.1 | 0.1 | 0.1 | EPA[292] |
a) |
A dash (-) indicates that the item is not applicable. |
(5) Propoxur, 14.7% Liquid (EC)
Six percent of the PM exposure interviews cited use of propoxur, 14.7% liquid EC (Table 13). Propoxur EC was used to control filth flies, sand flies, mosquitoes, and fleas, predominantly outdoors. Propoxur EC reportedly had a substantial level of use indoors as well (cited by 36% of PM exposure interviews), in structures such as mess and latrines. TIM 24 does not list propoxur EC; but it does list both indoor and outdoor uses for a propoxur, 70% solid (WP) and a 1% oil solution.[293] The 14.7% EC formulation (Baygon�) was ordered from the Defense Logistic Agency by units deploying to the KTO, and was reported by eleven Navy PM interviewees and three Army EAC interviewees. Table 59 presents the formulation-specific assumptions used for the application exposure assessment of propoxur, 14.7% liquid (EC).
Table 59. Propoxur, 14.7% liquid (EC) assumptions for applicationa
| Factor | Units | Definition/Explanation |
Assumptions by Level |
Source/Rationale | ||
|
Low |
Medium |
High |
||||
|
EP |
- | Exposure point | Outdoor | Indoor | Indoor | TIM24;[294] PM interviews |
|
VS |
gal/d | Volume of spray prepared daily | 1 | 5 | 15 | PM interviews; OPP/HED[295] |
|
FC |
% a.i. | Finished concentration | 1.0 | 1.0 | 4.5 | TIM 24[296] |
|
CS |
lb/gal | Concentration of active ingredient in formulation | 1.5 | 1.5 | 1.5 | Miles[297] |
|
VF |
gal/d | Volume of formulation used daily | 0.056 | 0.28 | 3.8 | Equation in text. |
|
WA |
lb a.i. /d | Weight of a.i. handled daily | 0.083 | 0.42 | 5.6 | Equation in text. |
|
ET |
h/d | Exposure time | 1 | 3 | 3 | PM interviews |
|
EF |
d/mo | Exposure frequency | 1 | 4 | 10 | PM interviews |
|
ED |
mo | Exposure duration | 1 | 4 | 7 | PM interviews |
|
ABS |
- | Dermal absorption factor | 0.16 | 0.16 | 0.16 | Hayes et al.[298] |
a) |
A dash (-) indicates that the item is not applicable. |
(6) EC Doses - Application
Table 60 presents doses potentially resulting from exposure during application of ECs. There are three types of doses presented for each of two cases; all for the evaluation of noncarcinogenic effects: PDRD, ADD, and PDRI. The two cases are when a handwand sprayer was used and when a backpack sprayer was used. Doses for the two cases differ somewhat for dermal exposure, but are identical for inhalation exposure because the respective UIE values listed in the PHED Guide are identical.
Table 60. ECs, dose rates - application, for evaluation of noncarcinogenic effectsa
| Formulation | Exposure Group | Exposure Point | ABS |
Handwand Sprayer |
Backpack Sprayer | ||||
| PDRD (mg/kg/d) | ADD (mg/kg/d) | PDRI (mg/kg/d) | PDRD (mg/kg/d) | ADD (mg/kg/d) | PDRI (mg/kg/d) | ||||
| Chlorpyrifos 45% liquid | Low | Outdoor | 0.03 | 2.56E-04 | 7.68E-06 | 1.79E-06 | 1.49E-03 | 4.47E-05 | 1.79E-06 |
| Medium | Outdoor | 0.03 | 1.28E-03 | 3.84E-05 | 8.94E-06 | 7.45E-03 | 2.23E-04 | 8.94E-06 | |
| High | Indoor | 0.03 | 2.38E+00 | 7.15E-02 | 7.15E-04 | 2.48E+00 | 7.43E-02 | 7.15E-04 | |
| Diazinon, 48% liquid | Low | Outdoor | 0.04 | 1.02E-04 | 4.10E-06 | 7.15E-07 | 5.96E-04 | 2.38E-05 | 7.15E-07 |
| Medium | Outdoor | 0.04 | 1.28E-03 | 5.12E-05 | 8.94E-06 | 7.45E-03 | 2.98E-04 | 8.94E-06 | |
| High | Indoor | 0.04 | 4.77E+00 | 1.91E-01 | 1.43E-03 | 4.96E+00 | 1.98E-01 | 1.43E-03 | |
| Malathion, 57% liquid | Low | Outdoor | 0.1 | 5.12E-04 | 5.12E-05 | 3.57E-06 | 2.98E-03 | 2.98E-04 | 3.57E-06 |
| Medium | Outdoor | 0.1 | 2.56E-03 | 2.56E-04 | 1.79E-05 | 1.49E-02 | 1.49E-03 | 1.79E-05 | |
| High | Indoor | 0.1 | 7.15E+00 | 7.15E-01 | 2.14E-03 | 7.43E+00 | 7.43E-01 | 2.14E-03 | |
| Propoxur, 14.7% liquid | Low | Outdoor | 0.16 | 5.12E-04 | 8.20E-05 | 3.57E-06 | 2.98E-03 | 4.77E-04 | 3.57E-06 |
| Medium | Indoor | 0.16 | 2.56E-03 | 4.10E-04 | 1.79E-05 | 1.49E-02 | 2.38E-03 | 1.79E-05 | |
| High | Indoor | 0.16 | 8.04E+00 | 1.29E+00 | 2.41E-03 | 8.36E+00 | 1.34E+00 | 2.41E-03 | |
|
|||||||||
a) |
ABS = dermal absorption factor. PDRD = potential dose rate for dermal contact. ADD = absorbed dermal dose. PDRI = potential dose rate for inhalation. UE = unit dermal exposure. WA = weight of a.i. handled BW = body weight. UIE = unit inhalation exposure. |
b) |
Formulas 1 and 3 adapted from the EPA, 1997.[299] |
Table 61 presents the application doses for the evaluation of carcinogenic effects
for propoxur EC. Carcinogenic activity for chlorpyrifos EC, diazinon EC,
and malathion EC is unlikely or unquantifiable (Tab D, Section D, "Toxicity
Assessment"). Table 61 presents two types of doses: LADDD and
LADDI. Once again, the dermal doses calculated for the handwand sprayer
differ from those calculated for the backpack sprayer, while the inhalation
doses are identical.
Table 61. ECs, lifetime average daily doses - application, for evaluation of carcinogenic effects
| Formulation | Exposure Group | Exposure Point |
Handwand Sprayer |
Backpack Sprayer |
||
| LADDD (mg/kg/d) | LADDI (mg/kg/d) | LADDD (mg/kg/d) | LADDI (mg/kg/d) | |||
| Chlorpyrifos, 45% liquidb | Low | Outdoor | - | - | - | - |
| Medium | Outdoor | - | - | - | - | |
| High | Indoor | - | - | - | - | |
| Diazinon, 48% liquidb | Low | Outdoor | - | - | - | - |
| Medium | Outdoor | - | - | - | - | |
| High | Indoor | - | - | - | - | |
| Malathion, 57% liquidb | Low | Outdoor | - | - | - | - |
| Medium | Outdoor | - | - | - | - | |
| High | Indoor | - | - | - | - | |
| Propoxur, 14.7% liquid | Low | Outdoor | - | - | - | - |
| Medium | Indoor | 2.57E-07 | 1.12E-08 | 1.49E-06 | 1.12E-08 | |
| High | Indoor | - | - | - | - | |
|
||||||
a) |
LADDD = lifetime average
daily absorbed dose via dermal contact. LADDI = lifetime average daily potential dose via inhalation. A dash (-) indicates that the item is not applicable. ADD = absorbed dermal dose. EF = exposure frequency. ED = exposure duration. AT = averaging time. PDRI = potential dose rate for inhalation. |
b) |
Carcinogenic activity unlikely or not quantifiable; see Toxicity Assessment. |
c) |
Formulas adapted from the EPA, 1997.[300] |
b. Post-Application Scenarios
(1) Common Elements
The post-application scenarios below address servicemembers who were exposed to ECs on surfaces inside mess and latrine facilities. In mess facilities, inhalation was the only consequential exposure route, while in latrines, both dermal and inhalation exposure were consequential. Air modeling for the post-application exposure to ECs is presented below. Investigators presume that opportunities for dermal contact in the mess were minimal, given that the only treated surfaces were normally limited to the intersections of walls and floors. The assumptions associated with the model mess and latrine structures are as follows:
Investigators estimated potential dermal post-application exposure to pesticide active ingredient residues on hard surfaces using a method provided by the EPA.[301] In the method, the amounts of residues transferred to hands, legs, and feet are calculated, being representative of areas that may have contacted treated surfaces in latrines. The actual parts exposed probably varied substantially. If servicemembers wore shoes, then feet were not exposed; however, the buttocks may have been exposed. At some camps, applicators may have treated shower areas, in which case feet might have been exposed. The assumptions provided by the EPA used for both ECs and the wettable powder (WP; bendiocarb) are presented in Table 62.
Table 62. ECs/WP, common assumptions for post application
| Factor (Units)a | Hands | Legsb | Feet | Source/Rationale |
| SA (cm2) | 840 | 5,050 | 1,120 | EPA[302] |
| TF | 12.6 | 2.4 | 13.6 | EPA[303] |
a) |
SA = surface area available for dermal contact; TF = transfer factor. |
b) |
Thighs and lower legs. |
Based on the PM exposure interviews, investigators presumed outdoor exposure
was inconsequential and so did not estimate it. Applicators sprayed ECs outdoors
around building and tent foundations, and around garbage containers. This would
have provided little or no opportunity for post-application exposure.
(2) Chlorpyrifos, 45% Liquid (EC)
Table 63 presents the formulation-specific assumptions investigators used for the post-application exposure assessment of chlorpyrifos, 45% liquid (EC). The table presents values for the high-exposure level only, since this level is associated with indoor exposure.
Table 63. Chlorpyrifos, 45% liquid (EC) assumptions for post applicationa
| Factor | Units | Definition/Explanation |
Assumptions by Level |
Source/Rationale | ||
|
Low |
Medium |
High |
||||
|
EP
|
- | Exposure point | Outdoor | Outdoor | Indoor | TIM24;[304] PM interviews |
|
AR
|
mg a.i. /cm2 | Application rate | - | - | 1.4E-02 | EPA[305] |
|
DT
|
mg a.i. /cm2 | Dislodgeable transferable residue; assumed to be 10% of the spray applied |
- |
- |
1.4E-03 |
EPA[306] |
|
CAM
|
mg/m3 | The a.i. air concentration in mess | - | - | 5.67E-04 | Air modeling |
|
CAL
|
mg/m3 | The a.i. Air concentration in latrine | - | - | 3.83E-03 | Air modeling |
|
ETM
|
h/d | Exposure time for mess | - | - | 2 | EPA[307] |
|
ETL
|
h/d | Exposure time for latrine | - | - | 0.5 | EPA[308] |
|
EF
|
d/mo | Exposure frequency | - | - | 30 | PM interviews |
|
ED
|
mo | Exposure duration | - | - | 8 | PM interviews |
|
ABS
|
- | Dermal absorption factor | - | - | 0.03 | ATSDR[309] |
a) |
A dash (-) indicates inconsequential exposure, or that the item is otherwise not applicable. |
(3) Diazinon, 48% Liquid (EC)
Table 64 presents the formulation-specific assumptions investigators used for the post-application exposure assessment of diazinon, 48% liquid (EC). The table presents values for the high exposure level only, since this level is associated with indoor exposure.
Table 64. Diazinon, 48% liquid (EC) assumptions for post applicationa
|
Factor
|
Units | Definition/Explanation |
Assumptions by Level |
Source/Rationale | ||
|
Low |
Medium |
High |
||||
|
EP
|
- | Exposure point | Outdoor | Outdoor | Indoor | TIM24;[310] PM interviews |
|
AR
|
mg a.i. /cm2 | Application rate | - | - | 2.8E-02 | EPA[311] |
|
DT
|
mg a.i. /cm2 | Dislodgeable transferable residue; assumed to be 10% of the spray applied | - | - | 2.8E-03 | EPA[312] |
|
CAM
|
mg/m3 | The a.i. air concentration in mess | - | - | 6.76E-03 | Air modeling |
|
CAL
|
mg/m3 | The a.i. air concentration in latrine | - | - | 4.57E-02 | Air modeling |
|
ETM
|
h/d | Exposure time for mess | - | - | 2 | EPA[313] |
|
ETL
|
h/d | Exposure time for latrine | - | - | 0.5 | EPA[314] |
|
EF
|
d/mo | Exposure frequency | - | - | 30 | PM interviews |
|
ED
|
mo | Exposure duration | - | - | 9 | PM interviews |
|
ABS
|
- | Dermal absorption factor | - | - | 0.04 | ATSDR[315] |
a) |
A dash (-) indicates inconsequential exposure, or that the item is otherwise not applicable. |
(4) Malathion, 57% Liquid (EC)
Table 65 presents the formulation-specific assumptions investigators used for the post-application exposure assessment of malathion, 57% liquid (EC). The table presents values for the high exposure level only, since this level is associated with indoor exposure.
Table 65. Malathion, 57% liquid (EC) assumptions for post applicationa
| Factor | Units | Definition/Explanation |
Assumptions by Level |
Source/Rationale | ||
| Low | Medium | High | ||||
|
EP
|
- | Exposure point | Outdoor | Outdoor | Indoor | TIM24;[316] PM interviews |
|
AR
|
mg a.i. /cm2 | Application rate | - | - | 5.6E-02 | EPA[317] |
|
DT
|
mg a.i. /cm2 | Dislodgeable transferable residue; assumed to be 10% of the spray applied | - | - | 5.6E-03 | EPA[318] |
|
CAM
|
mg/m3 | The a.i. air concentration in mess | - | - | 4.42E-03 | Air modeling |
|
CAL
|
mg/m3 | The a.i. air concentration in latrine | - | - | 2.99E-02 | Air modeling |
|
ETM
|
h/d | Exposure time for mess | - | - | 2 | EPA[319] |
|
ETL
|
h/d | Exposure time for latrine | - | - | 0.5 | EPA[320] |
|
EF
|
d/mo | Exposure frequency | - | - | 30 | PM interviews |
|
ED
|
mo | Exposure duration | - | - | 8 | PM interviews |
|
ABS
|
- | Dermal absorption factor | - | - | 0.1 | EPA[321] |
a) |
A dash (-) indicates inconsequential exposure, or that the item is otherwise not applicable. |
(5) Propoxur, 14.7% Liquid (EC)
Table 66 presents the formulation-specific assumptions investigators used for the post-application exposure assessment of propoxur, 14.7% liquid (EC). The table presents values for both the medium- and high-exposure levels, since both are associated with indoor exposure.
Table 66. Propoxur, 14.7% liquid (EC) assumptions for post applicationa
|
Factor
|
Units | Definition/Explanation |
Assumptions by Level |
Source/Rationale | ||
| Low | Medium | High | ||||
|
EP
|
- | Exposure point | Outdoor | Indoor | Indoor | TIM24;[322] PM interviews |
|
AR
|
mg a.i./cm2 | Application rate | - | 2.8E-02 | 1.3E-01 | EPA[323] |
|
DT
|
mg a.i./cm2 | Dislodgeable transferable residue; assumed to be 10% of the spray applied | - | 2.8E-03 | 1.3E-02 | EPA[324] |
|
CAM
|
mg/m3 | The a.i. air concentration in mess | - | 8.59E-05 | 1.72E-03 | Air modeling |
|
CAL
|
mg/m3 | The a.i. air concentration in latrine | - | 5.80E-04 | 1.16E-02 | Air modeling |
|
ETM
|
h/d | Exposure time for mess | - | 2 | 2 | EPA[325] |
|
ETL
|
h/d | Exposure time for latrine | - | 0.5 | 0.5 | EPA[326] |
|
EF
|
d/mo | Exposure frequency | - | 4 | 10 | PM interviews |
|
ED
|
mo | Exposure duration | - | 3 | 7 | PM interviews |
|
ABS
|
- | Dermal absorption factor | - | 0.16 | 0.16 | Hayes et al.[327] |
a) |
A dash (-) indicates inconsequential exposure, or that the item is otherwise not applicable. |
(6) Air Modeling for ECs
Investigators conducted indoor air modeling to estimate indoor concentrations of emulsifiable concentrates that might occur as a result of crack and crevice treatment. Investigators estimated post-application air equilibrium concentrations for a model mess building and a model latrine. A description of the model mess and latrine is provided in Tab D, Section C.7.b.(a), "Common Elements," above.
Investigators calculated long-term average emission rates for pesticide active ingredients offgassing from treated surfaces using an empirical relationship for evaporation time based on the vapor pressure and molecular weight of the active ingredient by the method of Chinn, as recommended by the EPA.[328] This relationship is based on the evaporation of pure substances under artificial conditions and may overestimate the emissions from substances in mixtures.
In this approach, investigators first calculated the Chinn evaporation time using the following formula:
te = 145/[(mw)(vp)]0.9546
where,
te = Chinn evaporation time (h)
mw = molecular weight of pesticide active ingredient (unitless)
vp = vapor pressure of pesticide active ingredient (mm Hg)
Next, investigators calculated a long-term emission rate using the following formula:
E = mass/te
where,
E = emission rate (mg/h)
mass = mass of active ingredient (mg)
Investigators calculated the mass of active ingredient for each scenario by multiplying the application rate (mass per area) by the application area. Investigators assumed the resulting constant emission rate to apply for the duration of the calculated evaporation time. In effect, investigators calculated concentrations for a single event.
The possible cumulative effects on airborne concentrations due to repeated applications on emission rate are not included in the calculation of concentrations. Investigators assumed that repeated applications to the same treatment area will largely restore or maintain the long-term average emission rate.
Investigators calculated indoor concentrations using a standard box model approach. One can develop the box model equation from the same mass balance considerations as described for permethrin, and employ the following differential equation:
V(dCAin/dt) = E + CAoutIV - CAinIV - K(CAin)V
where,
CAin = final indoor concentration (mg/m3)
CAout = ambient (outdoor) concentration (mg/m3)
E = emission rate (mg/h)
I = air changes per hour in room
V = room volume (m3)
t = time (h)
K = decay rate (h-1)
This equation has the following general solution:
CAin = [1/(I+K)][(E/V) + (CAout)(I)][1 - exp{-(I+K)(t)}] + CA0 exp{-(I+K)(t)}
where,
CA0 = initial concentration in room (mg/m3)
Investigators assumed: 1) the active ingredients to be nonreactive (K = 0), and 2) contributions from outdoors to be negligible (CAout = 0); the rationale is provided under permethrin.
With these assumptions, the equation for concentration within the room simplifies to:
CAin = [E/(I)(V)][1 - exp{-(I)(t)}] + CA0 exp{-(I)(t)}
For an initial concentration of zero (CA0 = 0), this equation for concentration at time t simplifies to the following expressions:
CAin = [E/(I)(V)][1 - exp{-(I)(t)}]
This equation yields estimated concentrations that asymptotically approach an equilibrium concentration given by:
CA = E/[(I)(V)]
The time in hours (tf) required to reach a given fraction (f) of the equilibrium concentration is:
tf = -[ln(1-f)]/I
Although the EPA recommends the use of the MCCEM in conducting high-end exposure assessments, investigators used the simple box model for the same reasons discussed under permethrin. Investigators modeled each structure examined for the application scenarios under consideration here (a mess and latrine) as a single chamber structure.
Investigators assumed ventilation rates of 2 and 4 air changes per hour for the high and medium indoor inhalation exposure scenarios, respectively, where applicable. Investigators believe these rates to be reasonable given reports that strong winds readily penetrated the structures under consideration.
For a ventilation rate of 2 air changes per hour, investigators estimated indoor concentrations to reach 50% of the equilibrium concentration in about 0.35 hours, 90% in 1.15 hours, 95% in 1.5 hours, and 99% in 2.3 hours. For a ventilation rate of 4 air changes per hour, the indoor concentrations are estimated to reach 50% of the equilibrium concentration in 0.17 hours, 90% in 0.58 hours, 95% in 0.75 hours, and 99% in 1.15 hours.
In accordance with draft the EPA guidance for conducting residential exposure assessments, investigators also calculated saturation concentrations to ensure that the estimated indoor concentrations do not exceed the associated saturation concentration. Investigators estimated saturation concentrations using the following equation:
CAsat = (VP/760)(mw)(106)/[(R)(T)] where,
vp = vapor pressure (mm Hg)
mw = molecular weight (g/mole)
R = 0.0821 liter atmosphere/mole oK, the universal gas constant
T = temperature of air (OK)
Investigators assumed an ambient temperature of 293o K (20o C) for the calculation of the saturation concentrations. The sensitivity of saturation concentration to temperature is relatively low over the range of temperatures one would expect inside a mess or latrine, since the saturation concentration is inversely proportional to the absolute temperature.
Calculated air equilibrium concentrations of ECs for the exposure scenarios of interest are summarized in Table 67. More detailed tables documenting the calculation of equilibrium concentrations and saturation concentrations for each EC and exposure scenario appear in Tables 68 through 71. The tables present the saturation concentrations solely to ensure that the estimated equilibrium concentrations do not exceed the corresponding saturation values. The saturation concentrations should not be used to represent ambient levels. In all cases, the estimated equilibrium concentrations are well below the associated saturation values.
Table 67. Post-application air equilibrium concentrations for ECs
| Active Ingredient | Exposure Site | Scenario |
Equilibrium Concentration (mg/m3) |
| Chlorpyrifos, 45% liquid (EC) |
Mess | High exposure | 5.67E-04 |
| Latrine | High exposure |
3.83E-03 |
|
| Diazinon, 48% liquid (EC) |
Mess | High exposure |
6.76E-03 |
| Latrine | High exposure |
4.57E-02 |
|
| Malathion, 57% liquid (EC) |
Mess | High exposure |
4.42E-03 |
| Latrine | High exposure |
2.99E-02 |
|
| Propoxur, 14.7% liquid (EC) |
Mess | Medium exposure |
8.59E-05 |
| High exposure |
1.72E-03 |
||
| Latrine | Medium exposure |
5.80E-04 |
|
| High exposure |
1.16E-02 |
As discussed previously, the modeled constant emission rate for each EC is based
on a Chinn evaporation calculation recommended by the EPA and represents emissions
for a single event. Although some increase in the effective emission rate might
occur as a result of the cumulative effects from repeated applications, there
are no on-site measurements available to quantify this effect. As stated previously,
investigators assumed that repeated applications to the same treatment area
restore or maintain the long-term calculated emission rates.
Table 68. Calculation of indoor air concentrations for chlorpyrifos, 45% liquid (EC)
| Location | Mess | Latrine |
| Exposure Scenario | High | High |
| Molecular Weight a.i. (g/mole) | 350.59 | 350.59 |
| Vapor pressure a.i. (mm Hg) | 1.87E-05 | 1.87E-05 |
| Application Rate (mg a.i./cm2) | 1.40E-02 | 1.40E-02 |
| Application Area (ft2) | 608 | 78 |
| Mass a.i. per application (mg) | 7907.91 | 1014.50 |
| Room volume (ft3) | 14000 | 266 |
| Room volume (m3) | 396.44 | 7.53 |
| Air changes per hour | 2 | 2 |
| Ventilation rate (ft3/h) | 28000 | 532 |
| Ventilation rate (m3/h) | 792.87 | 15.06 |
| Chinn evaporation time (h) | 1.76E+04 | 1.76E+04 |
| Chinn evaporation rate (mg/h) | 4.49E-01 | 5.76E-02 |
| Equilibrium concentration (mg/m3) a | 5.67E-04 | 3.83E-03 |
| Saturation concentration (mg/m3) | 3.59E-01 | 3.59E-01 |
a) |
Equilibrium concentration is bold because it is the air concentration used to calculate doses. |
Table 69. Calculation of indoor air concentrations for diazinon, 48% liquid (EC)
| Location | Mess | Latrine |
| Exposure Scenario | High | High |
| Molecular Weight a.i. (g/mole) | 304.36 | 304.36 |
| Vapor pressure a.i. (Mm Hg) | 1.40E-04 | 1.40E-04 |
| Application Rate (mg a.i./cm2) | 2.80E-02 | 2.80E-02 |
| Application Area (ft2) | 608 | 78 |
| Mass a.i. per application (mg) | 15815.81 | 2029.00 |
| Room volume (ft3) | 14000 | 266 |
| Room volume (m3) | 396.44 | 7.53 |
| Air changes per hour | 2 | 2 |
| Ventilation rate (ft3/h) | 28000 | 532 |
| Ventilation rate (m3/h) | 792.87 | 15.06 |
| Chinn evaporation time (h) | 2.95E+03 | 2.95E+03 |
| Chinn evaporation rate (mg/h) | 5.36E+00 | 6.88E-01 |
| Equilibrium concentration (mg/m3)a | 6.76E-03 | 4.57E-02 |
| Saturation concentration (mg/m3) | 2.33E+00 | 2.33E+00 |
|
a) |
Equilibrium concentration is bold because it is the air concentration used to calculate doses. |
Table 70. Calculation of indoor air concentrations for malathion, 57% liquid
(EC)
| Location | Mess | Latrine |
| Exposure Scenario | High | High |
| Molecular Weight a.i. (g/mole) | 330.36 | 330.36 |
| Vapor pressure a.i. (Mm Hg) | 4.00E-05 | 4.00E-05 |
| Application Rate (mg a.i./cm2) | 5.60E-02 | 5.60E-02 |
| Application Area (ft2) | 608 | 78 |
| Mass a.i. per application (mg) | 31631.63 | 4058.00 |
| Room volume (ft3) | 14000 | 266 |
| Room volume (m3) | 396.44 | 7.53 |
| Air changes per hour | 2 | 2 |
| Ventilation rate (ft3/h) | 28000 | 532 |
| Ventilation rate (m3/h) | 792.87 | 15.06 |
| Chinn evaporation time (h) | 9.02E+03 | 9.02E+03 |
| Chinn evaporation rate (mg/h) | 3.51E+00 | 4.50E-01 |
| Equilibrium concentration (mg/m3)a | 4.42E-03 | 2.99E-02 |
| Saturation concentration (mg/m3) | 7.23E-01 | 7.23E-01 |
|
a) |
Equilibrium concentration is bold because it is the air concentration used to calculate doses. |
Table 71. Calculation of indoor air concentrations for propoxur, 14.7% liquid
(EC)
| Location | Mess | Mess | Latrine | Latrine |
| Exposure Scenario | High | Medium | High | Medium |
| Molecular Weight a.i. (g/mole) | 209.24 | 209.24 | 209.24 | 209.24 |
| Vapor pressure a.i. (Mm Hg) | 9.70E-06 | 9.70E-06 | 9.70E-06 | 9.70E-06 |
| Application Rate (mg a.i./cm2) | 1.30E-01 | 1.30E-02 | 1.30E-01 | 1.30E-02 |
| Application Area (ft2) | 608 | 608 | 78 | 78 |
| Mass a.i. per application (mg) | 73430.56 | 7343.06 | 9420.37 | 942.04 |
| Room volume (ft3) | 14000 | 14000 | 266 | 266 |
| Room volume (m3) | 396.44 | 396.44 | 7.53 | 7.53 |
| Air changes per hour | 2 | 4 | 2 | 4 |
| Ventilation rate (ft3/h) | 28000 | 56000 | 532 | 1064 |
| Ventilation rate (m3/h) | 792.87 | 1585.74 | 15.06 | 30.13 |
| Chinn evaporation time (h) | 5.39E+04 | 5.39E+04 | 5.39E+04 | 5.39E+04 |
| Chinn evaporation rate (mg/h) | 1.36E+00 | 1.36E-01 | 1.75E-01 | 1.75E-02 |
| Equilibrium concentration (mg/m3)a | 1.72E-03 | 8.59E-05 | 1.16E-02 | 5.80E-04 |
| Saturation concentration (mg/m3) | 1.11E-01 | 1.11E-01 | 1.11E-01 | 1.11E-01 |
|
a) |
Equilibrium concentration is bold because it is the air concentration used to calculate doses. |
Information in the literature about studies involving the four ECs under consideration here show that although ECs may remain active for extended periods of time following indoor application, the measured airborne indoor concentrations generally decrease fairly rapidly with half-life values on the order of several hours to 1.5 days. Therefore, the use of an assumed constant, long-term average emission rate (without the incorporation of any consideration of decay) should, on balance, provide a conservative estimate of equilibrium concentrations.
Average airborne concentrations of chlorpyrifos measured in dormitory rooms receiving spray applications to cracks and crevices were 100, 1100, 1100, 800, and 300 ng/m3 before treatment, immediately after treatment, 1 day after, 2 days after, and 3 days after treatment, respectively.[329] In another study, airborne concentrations of chlorpyrifos receiving spray or aerosol application to cracks and crevices decreased from 2700 ng/m3 immediately following application to 50 ng/m3 3 days later.[330] In a third study, airborne levels of chlorpyrifos following crack and crevice treatment in food preparation areas ranged from 20 - 1488 ng/m3 immediately after spraying to 4 - 361 ng/m3 after 24 hours.[331]
If chlorpyrifos is released to the air in vapor phase, it will react with photochemically-produced hydroxyl radicals at an estimated half life of approximately 6.34 hours.[332] Diazinon has an estimated vapor phase half-life in the atmosphere of 4.1 hours due to reactions with photochemically produced hydroxyl radicals.[333] Malathion has an estimated vapor phase half life in the atmosphere of 1.5 days resulting from hydrogen abstraction by photochemically produced hydroxyl radicals.[334]
One study measured airborne concentrations after application of propoxur to a dormitory room.[335] Concentrations decreased from 15.4 mg/m3 following application, to 2.7 mg/m3 after 1 day, to 1.8 m g/m3 after 2 days, and to 0.7 mg/m3 after 3 days. Propoxur is primarily released to the atmosphere in the form of an aerosol during its use as an insecticide and would subsequently be subject to gravitational settling. The resulting vapor phase propoxur will react with photochemically-produced hydroxyl radicals with a half-life of about 4.3 hours.[336] However, one would expect the half-life at indoor locations to be longer due to the generally lower concentration of hydroxyl radicals.
(7) EC Doses - Post Application
Table 72 presents doses potentially resulting from the post-application exposure to ECs. There are three types of doses presented for the evaluation of noncarcinogenic effects: PDRD, ADD, and PDRI. Table 73 presents the post-application doses for the evaluation of carcinogenic effects for propoxur EC. Carcinogenic activity for chlorpyrifos EC, diazinon EC, and malathion EC is unlikely or not quantifiable (Tab D, Section D, "Toxicity Assessment"). The two types of doses shown are LADDD and LADDI.
Table 72. ECs, dose rates - post application for evaluation of noncarcinogenic effectsa
|
Formulation |
Exposure Group |
Exposure Point |
ABS |
PDRD (mg/kg/d) |
ADD (mg/kg/d) |
PDRI (mg/kg/d) |
| Chlorpyrifos, 45% liquid | Low |
Outdoor |
0.03 |
- |
- |
- |
| Medium |
Outdoor |
0.03 |
- |
- |
- |
|
| High |
Indoor |
0.03 |
7.59E-01 |
2.28E-02 |
6.97E-05 |
|
| Diazinon, 48% liquid | Low |
Outdoor |
0.04 |
- |
- |
- |
| Medium |
Outdoor |
0.04 |
- |
- |
- |
|
| High |
Indoor |
0.04 |
1.52E+00 |
6.07E-02 |
8.31E-04 |
|
| Malathion, 57% liquid | Low |
Outdoor |
0.1 |
- |
- |
- |
| Medium |
Outdoor |
0.1 |
- |
- |
- |
|
| High |
Indoor |
0.1 |
3.03E+00 |
3.03E-01 |
5.44E-04 |
|
| Propoxur, 14.7% liquid | Low |
Outdoor |
0.16 |
- |
- |
- |
| Medium |
Indoor |
0.16 |
1.52E+00 |
2.43E-01 |
1.06E-05 |
|
| High |
Indoor |
0.16 |
7.05E+00 |
1.13E+00 |
2.11E-04 |
|
|
||||||
|
a) |
ABS = dermal
absorption factor. PDRD = potential dose rate for dermal contact (latrine only). ADD = absorbed dermal dose (latrine only). PDRI = potential dose rate for inhalation (mess and latrine). A dash (-) indicates that the item is not applicable. DT = dislodgeable transferable residue. TF = transfer factor. SA = surface area available for dermal contact. BW = body weight. CA = a.i. concentration in air from modeling (mess and latrine). IRA = inhalation rate. ET = exposure time (mess and latrine). |
|
b) |
Formula 1 is adapted from the EPA.[337] |
Table 73. ECs, lifetime average daily doses, post application for evaluation of carcinogenic effectsa
| Formulation | Exposure Group |
Exposure Point |
LADDD (mg/kg/d) |
LADDI (mg/kg/d) |
| Chlorpyrifos, 45% liquidb | Low | Outdoor | - | - |
| Medium | Outdoor | - | - | |
| High | Indoor | - | - | |
| Diazinon, 48% liquidb | Low | Outdoor | - | - |
| Medium | Outdoor | - | - | |
| High | Indoor | - | - | |
| Malathion, 57% liquidb | Low | Outdoor | - | - |
| Medium | Outdoor | - | - | |
| High | Indoor | - | - | |
| Propoxur, 14.7% liquid | Low | Outdoor | - | - |
| Medium | Indoor | 1.14E-04 | 4.69E-09 | |
| High | Indoor | - | - | |
|
||||
|
a) |
LADDD
= lifetime average daily absorbed dose via dermal contact. LADDI = lifetime average daily potential dose via inhalation. A dash (-) indicates that the item is not applicable. ADD = absorbed dermal dose. EF = exposure frequency. ED = exposure duration. AT = averaging time. PDRI = potential dose rate for inhalation. |
|
b) |
Carcinogenic activity unlikely or not quantifiable; Tab D, Section D, "Toxicity Assessment." |
|
c) |
Formulas adapted from the EPA, 1997.[338] |
8. Wettable Powder Used for Spraying
Investigators evaluated only bendiocarb, 76% solid, wettable powder (WP).
a. Application Scenarios
Six percent of the PM exposure interviews cited use of Bendiocarb WP (Table 13), which was used to control pests such as cockroaches, fleas, mosquitoes, ticks, and venomous arthropods. It was applied mainly by low-pressure 2-gallon handwand sprayers. Application appears to have been evenly split between outdoors and indoors. Outdoor applications were broadcast treatments typically made to the near-ground portions of buildings and tents, and other places where the target pests congregated. Indoor applications probably included crack and crevice treatment, as well as broadcast treatment of floors. During crack and crevice application, a stream or cone of spray is directed at cracks and crevices, such as commonly occur where a wall meets a floor. Investigators assumed surfaces adjacent to the cracks were treated, and that overspray could have been substantial. Additionally, building size, condition, and configuration impacted the relative fraction of surface area treated. Investigators assumed a much greater fraction of surface area was treated in a small latrine as opposed to a large rectangular mess hall. For this assessment, rigid structures serve as the models. Treatments inside tents probably occurred to some extent, but investigators did not specifically address this, as they had no reason to believe that the exposures would be any higher in tents versus rigid structures. The clothing scenarios and PPE levels used are identical to those described previously for ECs, and the associated assumptions are the same.
Compliance with PPE requirements may have been better for servicemembers applying bendiocarb WP than it was for servicemembers applying ECs. One hundred percent of servicemembers reportedly wore adequate PPE (Table 13). This is based on a relatively high proportion of servicemembers reporting (86%); however, only 14 interviewees provided information. Fourteen percent of interviewees (i.e., two interviewees) did not respond to this question. Furthermore, as with ECs, "adequate" PPE was not defined in the interviews. Finally, investigators assumed the most exposed were the least protected.
Table 74 presents the assumptions for application of bendiocarb WP. Investigators used the following equation[339] to calculate the weight of active ingredient handled daily:
WA = where,
WA = weight of active ingredient handled daily
VS = volume of spray prepared daily
FC = finished concentration
WD = density of water
CS = concentration of active ingredient in formulation
All other equations used in dose calculations are provided in the associated tables in the following subsections.
Table 74. Bendiocarb, 76% solid (WP) assumptions for applicationa
| Factor | Units | Definition/Explanation |
Assumptions by Level |
Source/Rationale | ||
|
Low |
Medium |
High |
||||
| EP | - | Exposure point | Outdoor | Indoor | Indoor | TIM24;[340] PM interviews |
| UE | mg/lb a.i. | Unit dermal exposure | 8.6 | 8.6 | 100[341] | 1998 PHED Guide: handwand sprayer[342] |
| UIE | mg/lb a.i. | Unit inhalation exposure | 0.11 | 0.11 | 1.1 | 1998 PHED Guide: handwand sprayer[343] |
| VS | gal/d | Volume of spray prepared daily | 1 | 5 | 15 | PM interviews[344] |
| FC | % a.i. | Finished concentration | 0.25 | 0.25 | 0.25 | TIM 24[345] |
| WD | lb/gal | Density of water | 8.34 | 8.34 | 8.34 | TIM 24[346] |
| CS | lb/lb | Concentration of active ingredient in formulation | 0.76 | 0.76 | 0.76 | Nor-Am[347] |
| WA | lb a.i./d | Weight of a.i. handled daily | 0.027 | 0.14 | 0.41 | Equation in text. |
| ET | h/d | Exposure time | 1 | 1 | 3 | PM interviews |
| EF | d/mo | Exposure frequency | 1 | 6 | 12 | PM interviews |
| ED | mo | Exposure duration | 1 | 4 | 7 | PM interviews |
| ABS | - | Dermal absorption factor | 0.06 | 0.06 | 0.06 | Hayes et al.[348] |
|
a) |
A dash (-) indicates inconsequential exposure, or that the item is otherwise not applicable. |
b. Bendiocarb Doses - Application
Table 75 presents doses potentially resulting from exposure during application of bendiocarb WP. Three types are presented for the evaluation of noncarcinogenic effects: PDRD, ADD, and PDRI. No doses are presented for the evaluation of carcinogenic effects, as such activity is unlikely with bendiocarb WP.
Table 75. Bendiocarb, dose rates - application, for evaluation of noncarcinogenic effectsa
| Formulation | Exposure Group | Exposure Point | ABS |
Handwand Sprayer |
||
| PDRD (mg/kg/d) | ADD (mg/kg/d) | PDRI (mg/kg/d) | ||||
| Bendiocarb, 76% Solid (WP) |
Low | Outdoor | 0.06 | 3.92E-02 | 2.35E-03 | 4.31E-04 |
| Medium | Indoor | 0.06 | 1.96E-01 | 1.18E-02 | 2.16E-03 | |
| High | Indoor | 0.06 | 5.88E-01 | 3.53E-02 | 6.47E-03 | |
|
||||||
|
a) |
ABS = dermal
absorption factor. PDRD = potential dose rate for dermal contact. ADD = absorbed dermal dose. PDRI = potential dose rate for inhalation. UE = unit dermal exposure. WA = weight of a.i. Handled BW = body weight. UIE = unit inhalation exposure. |
|
b) |
Formulas 1 and 3 adapted from the EPA, 1997.[349] |
c. Post-Application Scenarios
The post-application scenarios below address servicemembers who were exposed to bendiocarb WP on surfaces inside latrine facilities. Dermal exposure is the only consequential exposure route, as the vapor pressure of bendiocarb is 4.95E-06 mm Hg 25oC.[350] OPP typically evaluates inhalation for post-application exposure only for pesticide active ingredients having a vapor pressure of greater than or equal to 1E-05 mm Hg.[351] Investigators presumed opportunities for dermal contact in the mess to have been minimal, given that the only treated surfaces were normally limited to the intersections of walls and floors.
The application assumptions for the model mess and latrine facilities have been described previously (Tab D, Section C.7.b.(1), "Common Elements"). Other assumptions related to post-application dermal exposure also are the same as described previously for ECs.
As with ECs, outdoor exposure was not estimated, as investigators presumed it to have been inconsequential based on the PM exposure interviews. Bendiocarb WP was likely applied outdoors around building and tent foundations, and around garbage containers. This would have provided little or no opportunity for post-application exposure.
Table 76 presents the formulation-specific assumptions used for the post-application exposure assessment of bendiocarb, 76% solid (WP). Values are presented for the medium and high exposure levels associated with indoor exposure.
Table 76. Bendiocarb, 76% solid (WP) assumptions for post applicationa
| Factor | Units | Definition/Explanation |
Assumptions by Level |
Source/Rationale | ||
| Low | Medium | High | ||||
| EP | - | Exposure point | Outdoor | Indoor | Indoor | TIM 24;[352] PM interviews |
| AR | mg a.i. /cm2 | Application rate | - | 7E-03 | 7E-03 | EPA[353] |
| DT | mg a.i. /cm2 | Dislodgeable transferable residue; assumed to be 10% of the spray applied | - | 7E-04 | 7E-04 | EPA[354] |
| ETM | h/d | Exposure time for mess | - | 2 | 2 | EPA[355] |
| ETL | h/d | Exposure time for latrine | - | 0.5 | 0.5 | EPA[356] |
| EF | d/mo | Exposure frequency | - | 6 | 12 | PM interviews |
| ED | mo | Exposure duration | - | 4 | 7 | PM interviews |
| ABS | - | Dermal absorption factor | - | 0.06 | 0.06 | Hayes et al.[357] |
|
a) |
A dash (-) indicates inconsequential exposure, or that the item is otherwise not applicable. |
d. Bendiocarb Doses - Post Application
Table 77 presents doses potentially resulting from the post-application exposure to bendiocarb WP. There are two types of doses presented for the evaluation of noncarcinogenic effects: PDRD and ADD. No doses are presented for the evaluation of carcinogenic effects, as such activity is unlikely with bendiocarb WP.
Table 77. Bendiocarb, dose rates - post application, for evaluation of noncarcinogenic effectsa
|
Formulation |
Exposure Group |
Exposure Point |
ABS |
PDRD (mg/kg/d) |
ADD (mg/kg/d) |
| Bendiocarb, 76% solid (WP) |
Low | Outdoor | 0.06 | - | - |
| Medium | Indoor | 0.06 | 3.79E-01 | 2.28E-02 | |
| High | Indoor | 0.06 | 3.79E-01 | 2.28E-02 | |
|
|||||
|
a) |
ABS = dermal
absorption factor. PDRD = potential dose rate for dermal contact (latrine only). ADD = absorbed dermal dose (latrine only). A dash (-) indicates that the item is not applicable. DT = dislodgeable transferable residue. TF = transfer factor. SA = surface area available for dermal contact. BW = body weight. ET = exposure time (mess and latrine). |
|
b) |
Formula 1 is adapted from the EPA.[358] |
9. Products Used for Fogging
The term "fogging" as used here covers two distinct types: ultra low volume (ULV) application, and high volume (HV) application. Fogging was conducted throughout some camps mainly to control flying insects such as filth flies, sand flies, and mosquitoes. US servicemembers appear to have employed ULV application to a much greater extent than HV application, although both methods are discussed briefly below. However, investigators evaluated only ULV application in detail because there were few reports of HV application, none of which came from the survey or PM interviews.
ULV application disperses much less pesticide active ingredient than HV application. Armed Forces technical guidance[359] describes the ULV insecticide application process. The undiluted ULV insecticide formulation is dispersed from the truck sprayer as fine droplets (less than 50 microns diameter) mixed with air. The material discharged is a fine mist that does not obscure visibility. The truck travels at 5-10 miles per hour while dispensing the insecticide in a 300-foot-wide swath. The droplets must be generally in the 5-25 micron size range to be effective. Less than 10% of the droplets should be smaller than 5 microns (small droplets do not impinge on the insects and are not effective). ULV application may have also been made by backpack-mounted and/or hand-held equipment. ULVs were used predominantly outdoors (Table 13).
High volume fogging disperses more pesticide active ingredient. Additionally, HV equipment requires the use of "inert" carrier substances. For example, the pesticide active ingredient (e.g., malathion) may be diluted with diesel fuel or other petroleum-based solvent prior to discharge. Thermal fog applicators discharge pesticide product in a smoke.[360] The fog generated during HV application obscures visibility. Common dispersal equipment includes truck-mounted fog generators.
a. Application Scenarios
(1) Common Elements
Table 78 presents the common assumptions for ULV fog application. The unit exposure values presented are from the PHED Guide. All values used to calculate the doses to applicators are presented in the following subsections.
Table 78. ULV fogging, common assumptions for application
| Factor | Units | Definition/Explanation |
Assumptions by Level |
Source/Rationale | ||
| Low | Medium | High | ||||
| UE | mg/ lb a.i. |
Unit dermal exposure | 0.023 | 0.023 | 2.9 | 1998 PHED Guide: Aerosol[361] |
| UIE | mg/ lb a.i. |
Unit inhalation exposure | 0.00012 | 0.00012 | 0.0012 | 1998 PHED Guide: Aerosol[362] |
The PPE considerations and rationale are essentially the same as for ECs.
The percentile values listed for servicemembers wearing PPE on Table 13 cannot be taken at face value. For ULV fogs, the percentile values presented would seem to indicate that 75-100% of servicemembers wore "adequate" PPE; however, much uncertainty surrounds these values: 1) adequate PPE was not defined in the interviews; 2) 22-39% of servicemembers who cited ULV fogs did not answer the question on PPE; and 3) the data do not reflect qualitative statements provided by interviewees indicating that some applicators had little or no PPE.
One of the important pesticide active ingredient-specific assumptions needed to calculate dose is the amount of active ingredient handled daily (WA). In order to calculate WA, it is first necessary to estimate the area treated per hour (AH), as follows:
AH = (VT x SW x CF1)/CF2 = 273 ac/h
where,
VT = velocity of truck = 7.5 mi/h
SW = width of fog swath = 300 ft
CF1 = unit correction factor 1 = 5,280 ft/MI
CF2 = unit correction factor 2 = 43,560 ft2/ac
For the ULV fogging exposure assessment, AH is rounded to 275 ac/h. Then the area treated per day (AD) = AH x exposure time. All other equations used in dose calculations are provided in the associated tables in the following subsections.
(2) Chlorpyrifos, 19% Liquid (ULV)
Eleven percent of the PM exposure interviews cited use of chlorpyrifos, 19% liquid (ULV) (Table 13). Table 79 presents the formulation-specific assumptions used for the application exposure assessment of chlorpyrifos ULV.
Table 79. Chlorpyrifos ULV assumptions for applicationa
| Factor | Units | Definition/Explanation |
Assumptions by Level |
Source/Rationale | ||
| Low | Medium | High | ||||
| AD | ac/d | Area treated per day | 275 | 550 | 1,100 | ET x 275 ac/h (see text) |
| AR | fl oz/ac | Application rate of formulation | 1.6 | 1.6 | 1.6 | TIM 24[363] |
| V | gal/d | Volume handled daily | 3.44 | 6.88 | 13.8 | A x 0.0125 gal/ac |
| CS | lb a.i. /gal | Concentration of a.i. in formulation | 1.5 | 1.5 | 1.5 | Product label[364] |
| WA | lb a.i./d | Weight of a.i. handled daily | 5.2 | 10.3 | 21 | V x CS |
| ABS | - | Dermal absorption factor | 0.03 | 0.03 | 0.03 | ATSDR[365] |
| ET | h/d | Exposure time | 1 | 2 | 4 | PM interviews (Table 13) |
| EF | d/mo | Exposure frequency | 1 | 8 | 21 | PM interviews (Table 13) |
| ED | mo | Exposure duration | 1 | 4 | 8 | PM interviews (Table 13) |
|
a) |
A dash (-) indicates inconsequential exposure, or that the item is otherwise not applicable. |
(3) Malathion, 91% Liquid (ULV)
Thirteen percent of the PM exposure interviews cited use of malathion, 91% liquid (ULV) (Table 13). Table 80 presents the formulation-specific assumptions used for the application exposure assessment of malathion ULV.
Table 80. Malathion ULV assumptions for applicationa
| Factor | Units | Definition/Explanation |
Assumptions by Level |
Source/Rationale | ||
|
Low |
Medium |
High |
||||
| AD | ac/d | Area treated per day | 275 | 825 | 1,925 | ET x 275 ac/h (see text) |
| AR | fl oz/ac | Application rate of formulation | 8 | 8 | 8 | TIM 24[366] |
| V | gal/d | Volume handled daily | 17 | 52 | 120 | A x 0.0625 gal/ac |
| CS | lb a.i. /Gal | Concentration of a.i. in formulation | 9.33 | 9.33 | 9.33 | Product label[367] |
| WA | lb a.i./d | Weight of a.i. handled daily | 159 | 485 | 1,120 | V x CS |
| ABS | - | Dermal absorption factor | 0.1 | 0.1 | 0.1 | EPA[368] |
| ET | h/d | Exposure time | 1 | 3 | 7 | PM interviews (Table 13) |
| EF | d/mo | Exposure frequency | 1 | 10 | 30 | PM interviews (Table 13) |
| ED | mo | Exposure duration | 1 | 5 | 8 | PM interviews (Table 13) |
|
a) |
A dash (-) indicates inconsequential exposure, or that the item is otherwise not applicable. |
(4) ULV Doses - Application
Table 81 presents doses potentially resulting from exposure during application of ULV fogs. There are three types of doses presented for the evaluation of noncarcinogenic effects: PDRD, ADD, and PDRI. Toxicity values were not available for the assessment of the potential carcinogenic effects of chlorpyrifos and malathion (Tab D, Section D, "Toxicity Assessment"), so investigators did not calculate LADDs.
Table 81. ULVs, dose rates - application, for evaluation of noncarcinogenic effectsa
|
Formulation |
Exposure Group |
Exposure Point |
ABS |
PDRD (mg/kg/d) |
ADD (mg/kg/d) |
PDRI (mg/kg/d) |
| Chlorpyrifos,
19% liquid (ULV) |
Low | Outdoor | 0.03 | 1.71E-03 | 5.13E-05 | 8.91E-06 |
| Medium | Outdoor | 0.03 | 3.38E-03 | 1.02E-04 | 1.77E-05 | |
| High | Outdoor | 0.03 | 8.70E-01 | 2.61E-02 | 3.60E-04 | |
| Malathion,
91% liquid (ULV) |
Low | Outdoor | 0.1 | 5.22E-02 | 5.22E-03 | 2.73E-04 |
| Medium | Outdoor | 0.1 | 1.59E-01 | 1.59E-02 | 8.31E-04 | |
| High | Outdoor | 0.1 | 4.64E+01 | 4.64E+00 | 1.92E-02 | |
|
||||||
|
a) |
ABS
= dermal absorption factor. PDRD = potential dose rate for dermal contact. ADD = absorbed dermal dose. PDRI = potential dose rate for inhalation. |
UE
= unit dermal exposure. WA = weight of a.i. Handled BW = body weight. UIE = unit inhalation exposure. |
|
b) |
Formulas 1 and 3 adapted from the EPA, 1997.[369] | |